On October 13, 2021, the historical group of the Royal Society of Chemistry organised a symposium celebrating ~150 years of the history of (molecular) chirality. We met for the first time in person for more than 18 months and were treated to a splendid and diverse program about the subject. The first speaker was Professor John Steeds from Bristol, talking about the early history of light and the discovery of its polarisation. When a slide was shown about herapathite[1] my “antennae” started vibrating. This is a crystalline substance made by combining elemental iodine with quinine in acidic conditions and was first discovered by William Herapath as long ago as 1852[2] in unusual circumstances. Now to the serendipity!
Herapath was able to get small crystals of this substance and discovered that when he placed one crystal upon another at “right angles”, the combination went “black as midnight”. He recognised that it was functioning as an excellent linear light polarizer, absorbing virtually all the light polarized along the shorter axis of the best-developed facet of the crystal. A number of well known scientists investigated this substance at the time, but by about 1951 it had largely been forgotten. The person to rediscover it was Edwin Land, of Polaroid camera fame.[3] He oriented the microcrystals into an extruded polymer to stabilize them and hence produce the first large-aperture light polarizer, which enabled him to manufacture his first camera. The serendipity resulted from him spotting the by then forgotten properties of Herapathite (I wonder if he recorded how this actually came about) and recognising how to exploit it.
In 2009 Bart Kahr had noticed that the crystal structure of this material had never been reported. It was a challenging structure to solve[1] but established that the polarizing property of the crystals was in large measure due to the presence of infinite chains of I3– units aligned in an almost linear channel in the crystal structure. And so it was that in October 2021, John Steeds showed the structure containing these iodine chains in his slide on the topic. The crystal structure is in the CCDC database as WEYDOV and can be seen here at DOI: 10.5517/ccsdg7v I show below part of the extended lattice, showing that chain of iodines.
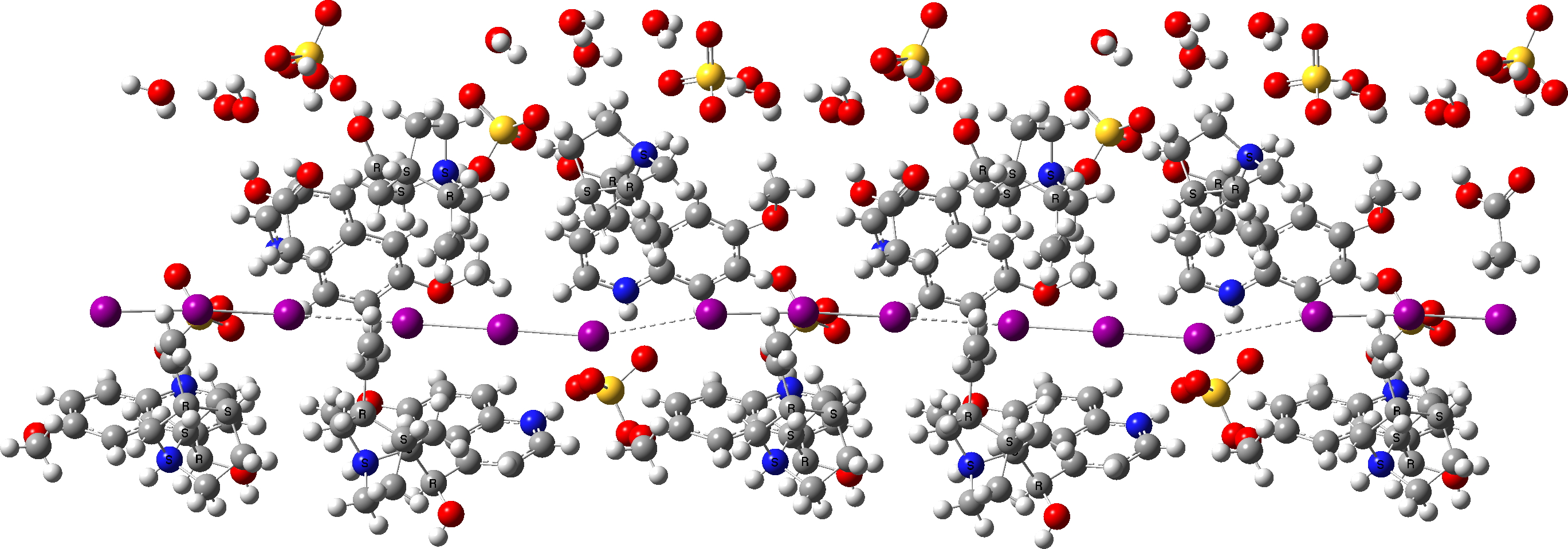
Click to view 3D model of WEYDOV
So the next (possible) instance of serendipity. From the audience, I immediately recognised this structural motif as being related to the crystal structure of both Na+I– (NAIACE) and Na+I2– (GADMOO)[4] which I discussed in one of the very first posts on this blog in 2009 as part of a story about the Finkelstein reaction. Both these structures were obtained from acetone solution, and this solvent very much forms part of the crystal structures, serving to coordinate the sodium cations and playing the role of the quinine in herapathite. The iodine chains, comprising in GADMOO units of I3– and I–, are almost exactly linear!
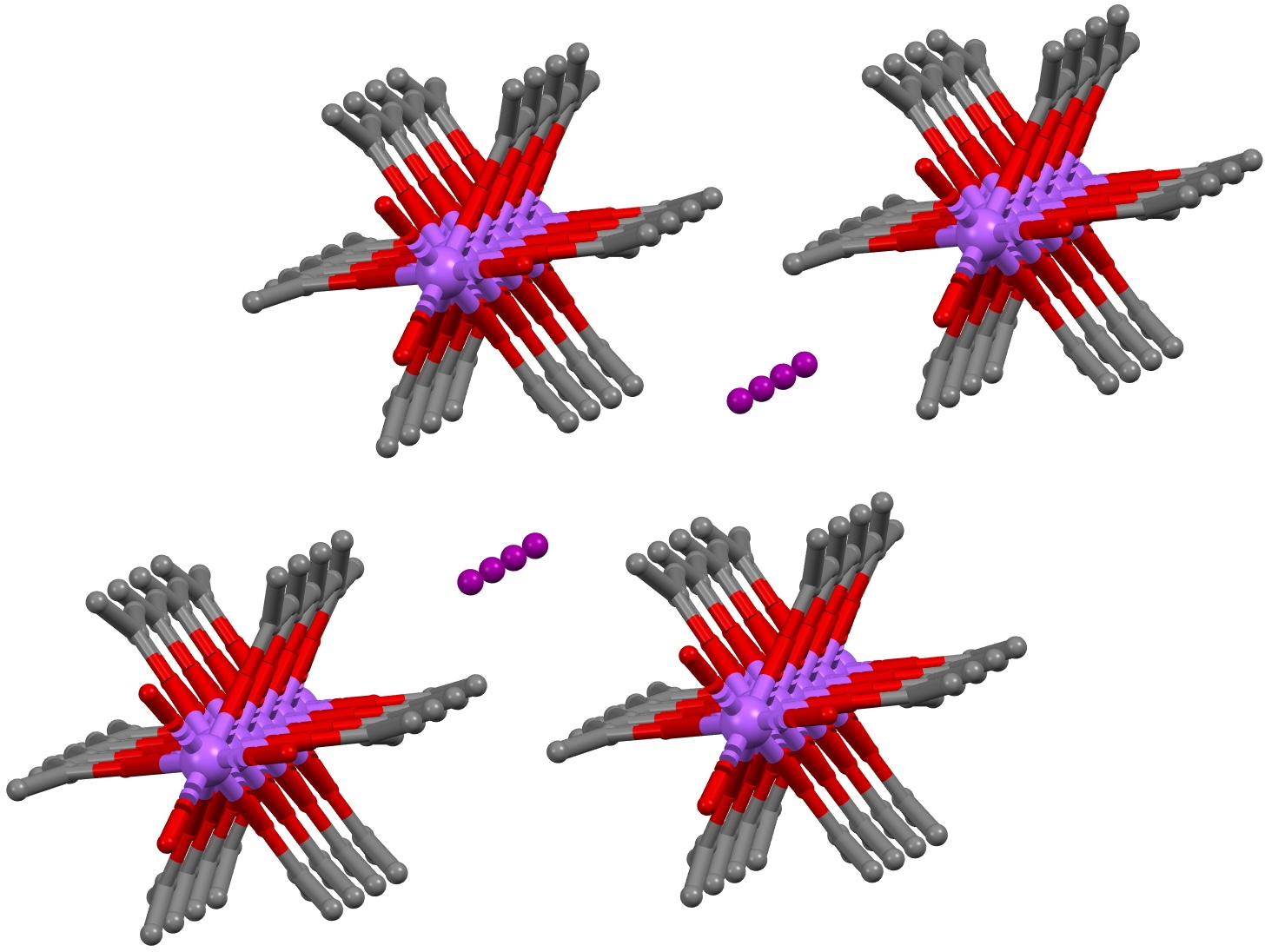
Click to view 3D model of NAICE
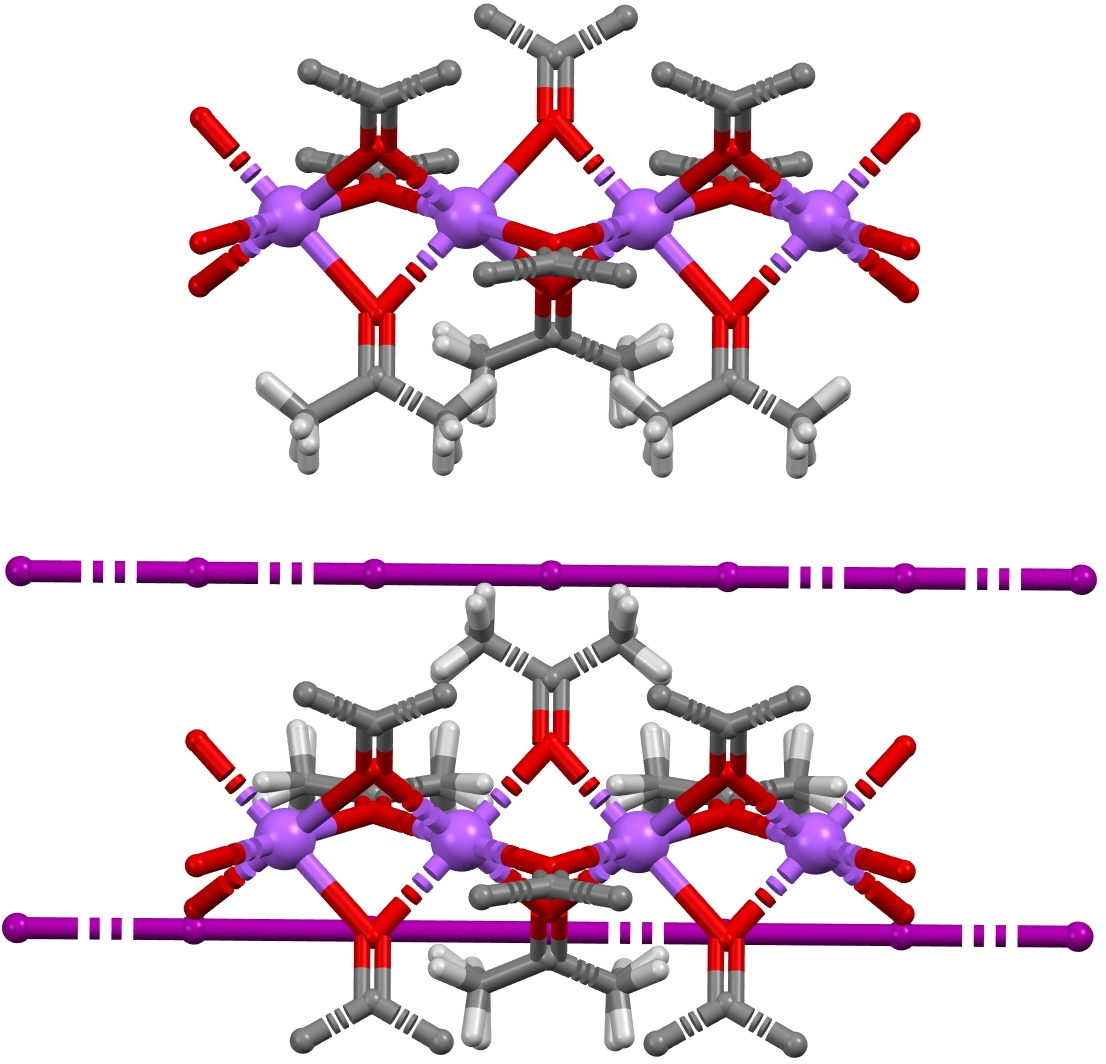
Click to view 3D model of GADMOO
So, the question arises as to whether crystals of Na+I2– have ever been examined for light polarisation? One might also ask whether eg the chiral quinine imparts a critical property to the herapathite crystal, or could the achiral acetone also serve the purpose? What would happen if substituted versions of acetone were used (halo, methyl etc)? Would they destroy those linear chains, or would they survive? Are repeating chains of I3– units essential, or can chains of alternating units of I3– and I– also serve the purpose? All questions that can only be answered by experiments! Anyone up for trying?
This post has DOI: 10.14469/hpc/9537
References
- B. Kahr, J. Freudenthal, S. Phillips, and W. Kaminsky, "Herapathite", Science, vol. 324, pp. 1407-1407, 2009. https://doi.org/10.1126/science.1173605
- W.B. Herapath, "XXVI. <i>On the optical properties of a newly-discovered salt of quinine, which crystalline substance possesses the power of polarizing a ray of light, like tourmaline, and at certain angles of rotation of depolarizing it, like selenite</i>", The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, vol. 3, pp. 161-173, 1852. https://doi.org/10.1080/14786445208646983
- E.H. Land, "Some Aspects of the Development of Sheet Polarizers*", Journal of the Optical Society of America, vol. 41, pp. 957, 1951. https://doi.org/10.1364/josa.41.000957
- R.A. Howie, and J.L. Wardell, "Polymeric tris(μ<sub>2</sub>-acetone-κ<sup>2</sup><i>O</i>:<i>O</i>)sodium polyiodide at 120 K", Acta Crystallographica Section C Crystal Structure Communications, vol. 59, pp. m184-m186, 2003. https://doi.org/10.1107/s0108270103006395