The homologous hydrocarbon series R4C is known for R=Me as neopentane and for R=Et as 3,3-diethylpentane. The next homologue, R=iPr bis(3,3-isopropyl)-2,4-dimethylpentane is also a known molecule[1] for which a crystal structure has been reported (DOI: https://doi.org/10.5517/cc4wvnh). The final member of the series, R= tbutyl is unknown. Here I have a look at some properties of the last two of these highly hindered hydrocarbons.
First the non-covalent-interactions (NCI) analysis, for a ωB97XD/Def2-SVPP/SCRF=dichloromethane wavefunction. This explores the properties of the weak electron density in the regions in between bonds, ie the non-bonded regions. In the presentation below, if that region is stabilizing, the surface is coloured cyan or dark green whereas if it is destabilising, it is pale green, yellow or orange.
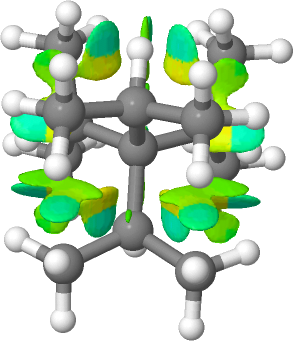
The above for R=iPr shows extensive but disconnected regions with NCI properties, with the pale cyan/green ones stabilizing and the regions verging on yellow repulsive. It would not be easy to conclude that this molecule overall is stabilised by dispersion! The predicted 1H NMR spectrum shows only one methine environment (2.59 ppm) but three methyl ones at 1.51, 1.04 and 0.94 (1.16 av/obs 2.23 and 1.04) ppm. The C-C bond lengths are 1.581Å (obs 1.599).
The NCI for R = tbutyl shows that the entire NCI surface is connected within the regions of the molecule, with far more green/yellow than stabilizing cyan. This molecule, which has an unusual T chiral symmetry is certainly sterically strained. The predicted C-C bond lengths of 1.668Å are unusually long (wB97XD/Def2-TZVPP).
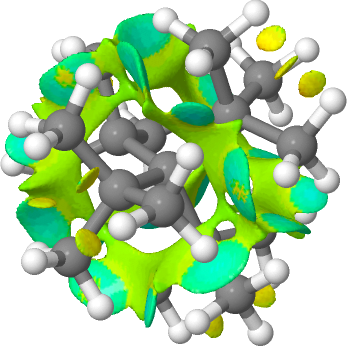
The optical rotation (589nm) is -179°, which raises the question of whether it would be configurationally stable? The predicted 1H NMR shows three methyl environments (2.21, 1.92 and 0.44 ppm), averaging to 1.52ppm, which is significantly different from the isopropyl analogue.
Tetra t-butylmethane is often cited as the smallest branched hydrocarbon that cannot be made.[2],[3],[4] Certainly it looks far more strained than the isopropyl version. Its preparation is a challenge that might never be achieved!
References
- S.I. Kozhushkov, R.R. Kostikov, A.P. Molchanov, R. Boese, J. Benet-Buchholz, P.R. Schreiner, C. Rinderspacher, I. Ghiviriga, and A. de Meijere, "Tetracyclopropylmethane: A Unique Hydrocarbon with S4 Symmetry", Angewandte Chemie International Edition, vol. 40, pp. 180-183, 2001. https://doi.org/10.1002/1521-3773(20010105)40:1<180::aid-anie180>3.0.co;2-k
- K.M. Nalin de Silva, and J.M. Goodman, "What Is the Smallest Saturated Acyclic Alkane that Cannot Be Made?", Journal of Chemical Information and Modeling, vol. 45, pp. 81-87, 2004. https://doi.org/10.1021/ci0497657
- M. Cheng, and W. Li, "Structural and Energetics Studies of Tri- and Tetra-<i>tert</i>-butylmethane", The Journal of Physical Chemistry A, vol. 107, pp. 5492-5498, 2003. https://doi.org/10.1021/jp034879r
- N.L. Allinger, J. Lii, and H.F. Schaefer, "Molecular Mechanics (MM4) Studies on Unusually Long Carbon–Carbon Bond Distances in Hydrocarbons", Journal of Chemical Theory and Computation, vol. 12, pp. 2774-2778, 2016. https://doi.org/10.1021/acs.jctc.5b00926