An earlier post investigated large anomeric effects involving two oxygen atoms attached to a common carbon atom.
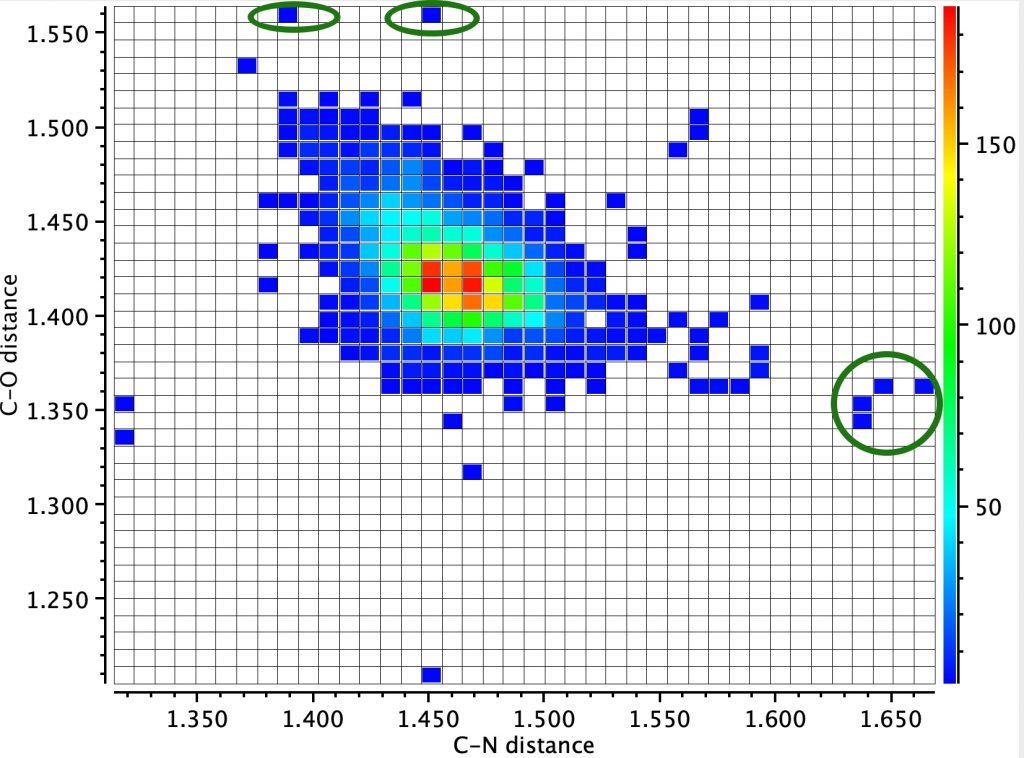
A variation is to replace one oxygen by a nitrogen atom, as in N-C-O. Shown below is a scatter plot of the two distances to the common carbon atom derived from crystal structures.
You can see some entries for which the C-O bond length is shorter than normal and the C-N distance very much longer than normal; an example of a highly asymmetric anomeric effect operating in just one direction rather than the two shown in the top diagram (red/blue arrows).
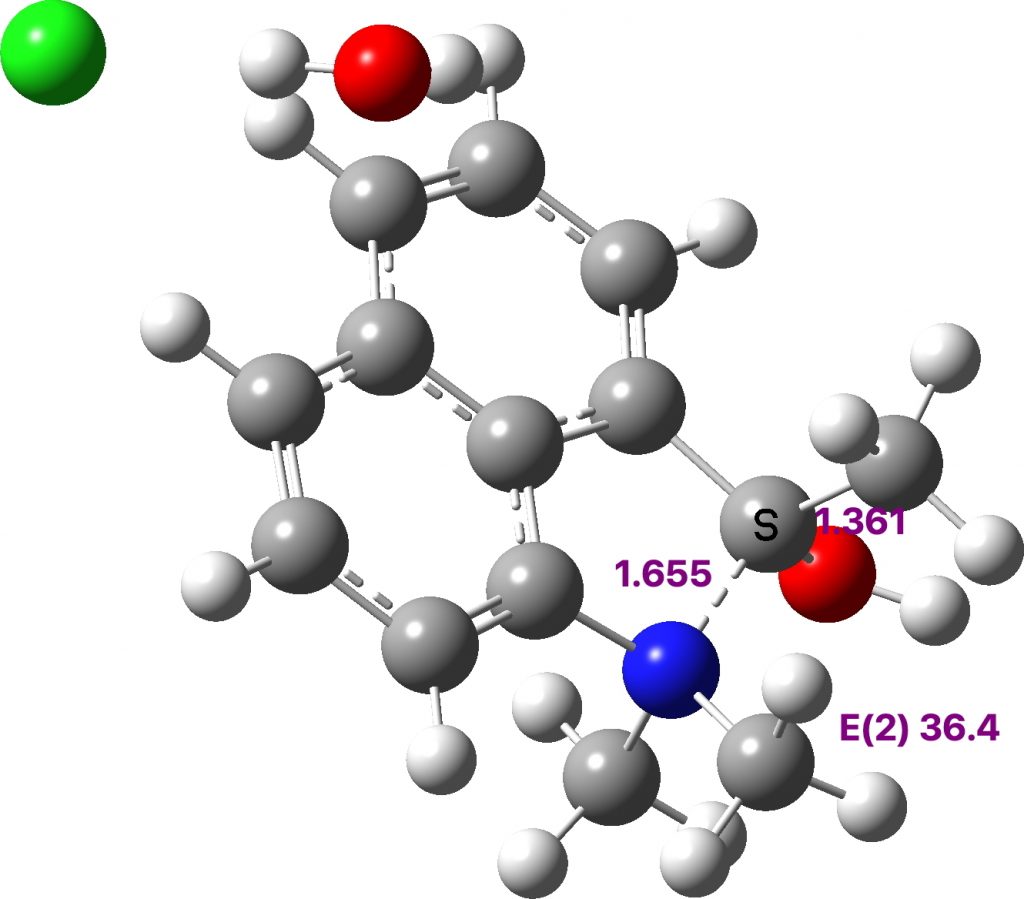
One example is LOFPON[1] (DOI: 10.5517/cc121rsn) with bond lengths shown calculated at the ωB97XD/def2svpp level (Calculation DOI: 10.14469/hpc/8682) and is rationalised by the nitrogen being a quaternary cation and hence an excellent leaving group which biases the electron flow towards it. Anomeric effects can be quantified using a technique known as NBO analysis, which uses perturbation theory to estimate the interaction energy between a donor orbital (the oxygen lone pair in this case) and an acceptor orbital (the C-N σ* unoccupied orbital). Populating the C-N σ* antibonding orbital causes the C-N length to increase and the interaction energy in this example is 36.4 kcal/mol. This is around twice the normal value for anomeric effects and so is unusually large.
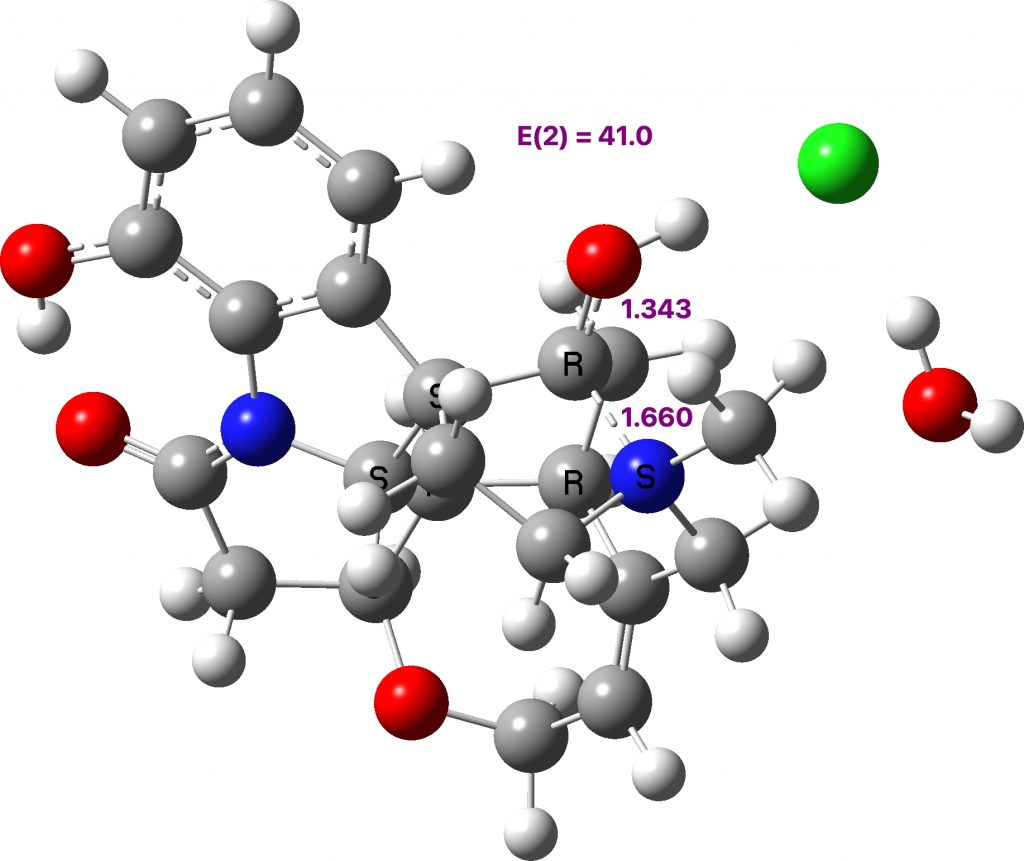
The other prominent example is NAWNUV (Data DOI: 10.5517/cc93pkm) where the bond length asymmetry is slightly larger and so is the perturbation energy (E2) is 41.0 kcal/mol (ωB97XD/def2svpp calculation DOI: 10.14469/hpc/8378).
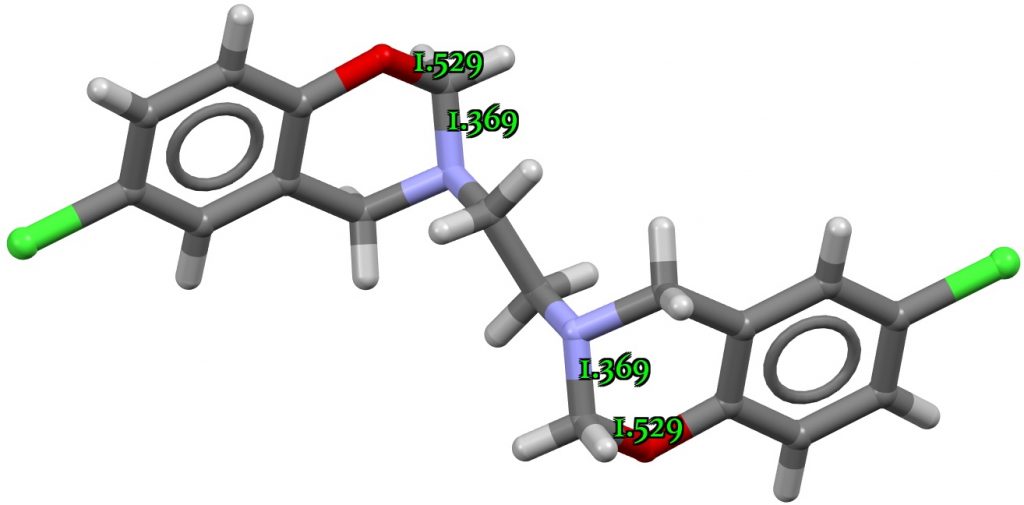
In the opposite direction, NUQKAM[2] is an example of a lengthened C-O bond and a shortened C-N bond, with the crystal structure (DOI: 10.5517/ccv3ln5) shown below.
In this instance, a ωB97XD/def2svpp calculation (Data DOI: 10.14469/hpc/8806) does not bear this structure out, with CN and CO bond lengths of 1.422 (vs 1.369) and 1.434 (vs 1.529)Å and a final E(2) of 22.1 kcal/mol (which is close to normal). This is an example of how mining the crystal structure can yield results that can be checked by a different (quantum computational) technique, which in this instance reveals a probable issue in the crystal structure refinement which is probably causing the apparently large anomeric effect in the crystal structure to manifest.
Another entry is ANUVUD[3] with a crystal structure (data DOI: 10.5517/ccdc.csd.cc24zxdg) shown below and CN and CO lengths of 1.391 and 1.559Å, which in this case ARE reasonably replicated by calculation (1.402, 1.499). This effect is promoted by the good leaving group ability of the carboxylate anion and the antiperiplanar orientation of the nitrogen lone pair with respect to the C-O bond, E(2)=35.2 kcal/mol (DOI: 10.14469/hpc/8807)
I end with FEHYOG, a relatively old structure[4] showing a very long C-N distance (1.673Å) but a normal associated C-O distance (1.423Å). This rings an alarm bell. Indeed, the respective computed distances are 1.482 and 1.425Å, a significant discrepancy (DOI: 10.14469/hpc/8769). The NBO interaction energy is an umremarkable 12.5 kcal/mol. 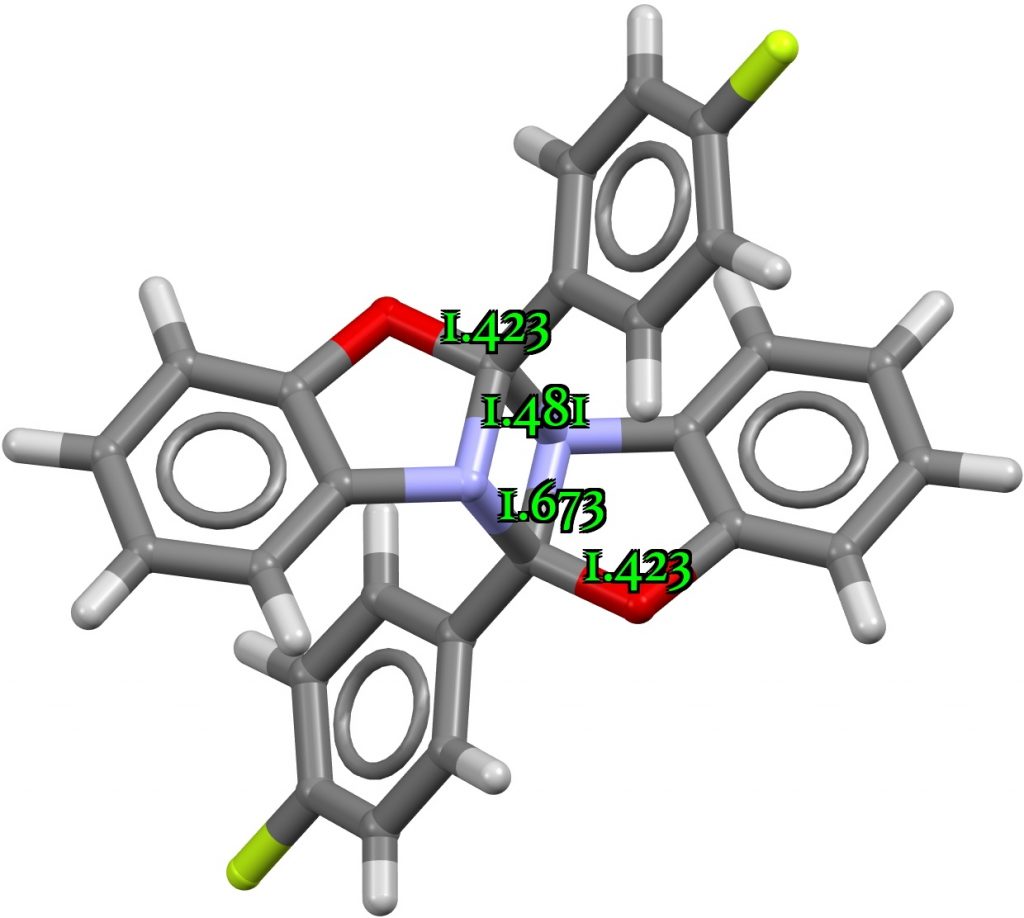
Data mining of the crystal structure database has revealed a number of abnormally large bond length asymmetries around the N-C-O unit. Some of these are true record breakers, but two have been identified where calculations cannot reproduce the observed bond lengths. One might indeed ask whether a quantum computation of the structure might not be added to the curation checks made by the CCDC of their database. It might improve the quality of the data even further!
References
- N. Mercadal, S.P. Day, A. Jarmyn, M.B. Pitak, S.J. Coles, C. Wilson, G.J. Rees, J.V. Hanna, and J.D. Wallis, "<i>O</i>-<i>vs. N</i>-protonation of 1-dimethylaminonaphthalene-8-ketones: formation of a<i>peri</i>N–C bond or a hydrogen bond to the pi-electron density of a carbonyl group", CrystEngComm, vol. 16, pp. 8363-8374, 2014. https://doi.org/10.1039/c4ce00981a
- A. Rivera, J.J. Rojas, J. Ríos-Motta, M. Dušek, and K. Fejfarová, "3,3′-Ethylenebis(3,4-dihydro-6-chloro-2<i>H</i>-1,3-benzoxazine)", Acta Crystallographica Section E Structure Reports Online, vol. 66, pp. o1134-o1134, 2010. https://doi.org/10.1107/s1600536810014248
- Y. Wang, D. Sun, Y. Chen, J. Xu, Y. Xu, X. Yue, J. Jia, H. Li, and L. Chen, "Alkaloids of Delphinium grandiflorum and their implication to H2O2-induced cardiomyocytes injury", Bioorganic & Medicinal Chemistry, vol. 37, pp. 116113, 2021. https://doi.org/10.1016/j.bmc.2021.116113
- N. Paillous, S.F. Forgues, J. Jaud, and J. Devillers, "[2 + 2] Cycloaddition of two CN double bonds. First structural evidence for head-to-tail photodimerization in the 2-phenylbenzoxazole series", J. Chem. Soc., Chem. Commun., pp. 578-579, 1987. https://doi.org/10.1039/c39870000578