Cyclopropenylidene must be the smallest molecule to be aromatic due to π-electrons, with just three carbon atoms and two hydrogen atoms. It has now been detected in the atmosphere of Titan, one of Saturn’s moons[1] and joins benzene, another aromatic molecule together with the protonated version of cyclopropenylidene, C3H3+ also found there.
The molecule has two π-electrons in the three membered ring and a carbene lone pair in the σ-framework. As with the cyclopropenium cation (C3H3+), these two electrons make it π-aromatic, as indicated by Hückel’s 4n+2 rule (n=0). I thought it might be fun to show the molecular orbitals containing these two pairs of electrons and then to show the result of a double excitation of the carbene lone pair into the π-system to make a anti-aromatic isomer with four π-electrons. This species is a whopping 209.3 kcal/mol higher in free energy, made up of the double electronic excitation energy topped up by conversion of the stabilizing aromaticity into destabilizing anti-aromaticity. Because of this antiaromaticity, the excited state is in fact a second order saddle point, avoiding anti-aromaticity by asymmetric distortion back down to the ground state and resymmetrisation.
| Ground state of Cyclopropenylidene | |
|---|---|
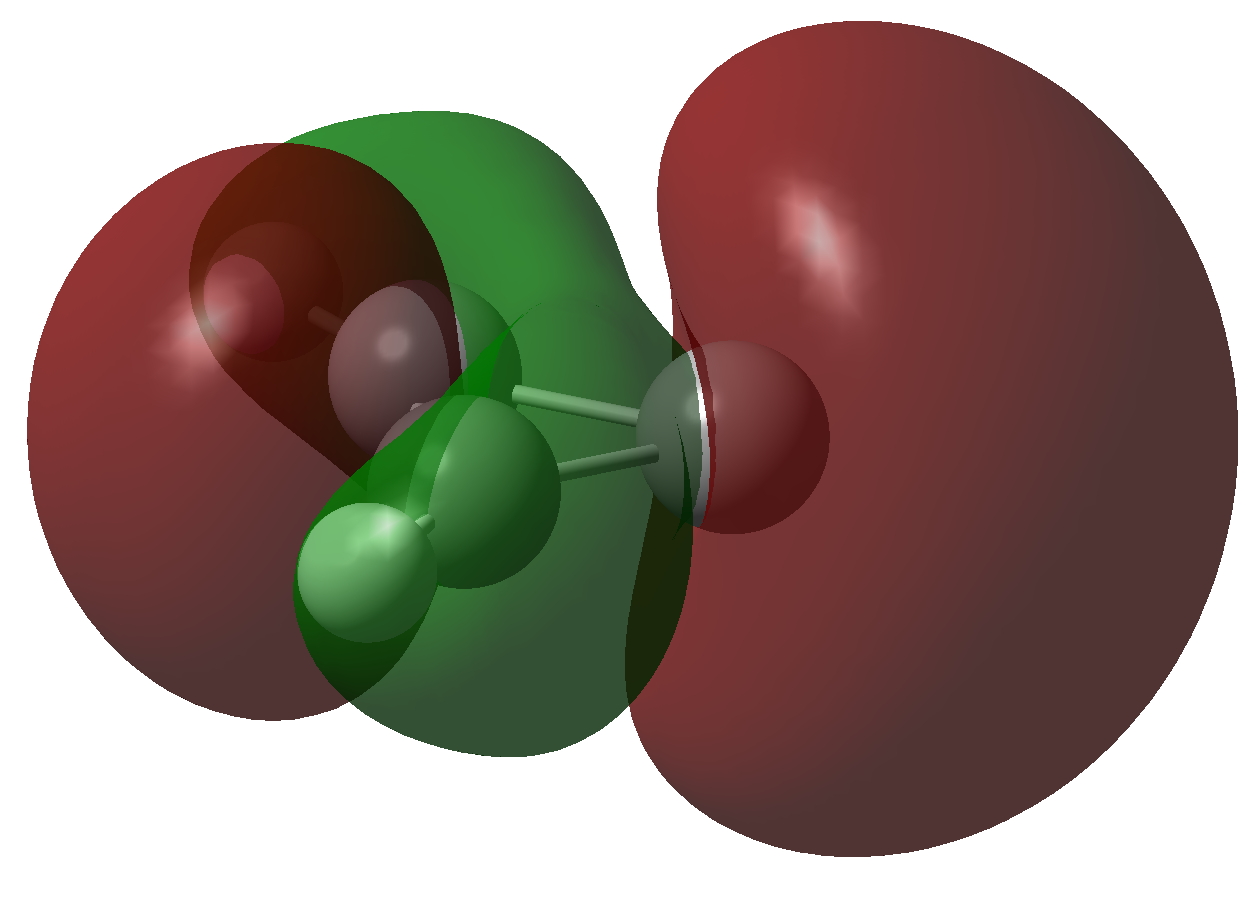 Click to view 3D model of NBO 10 |
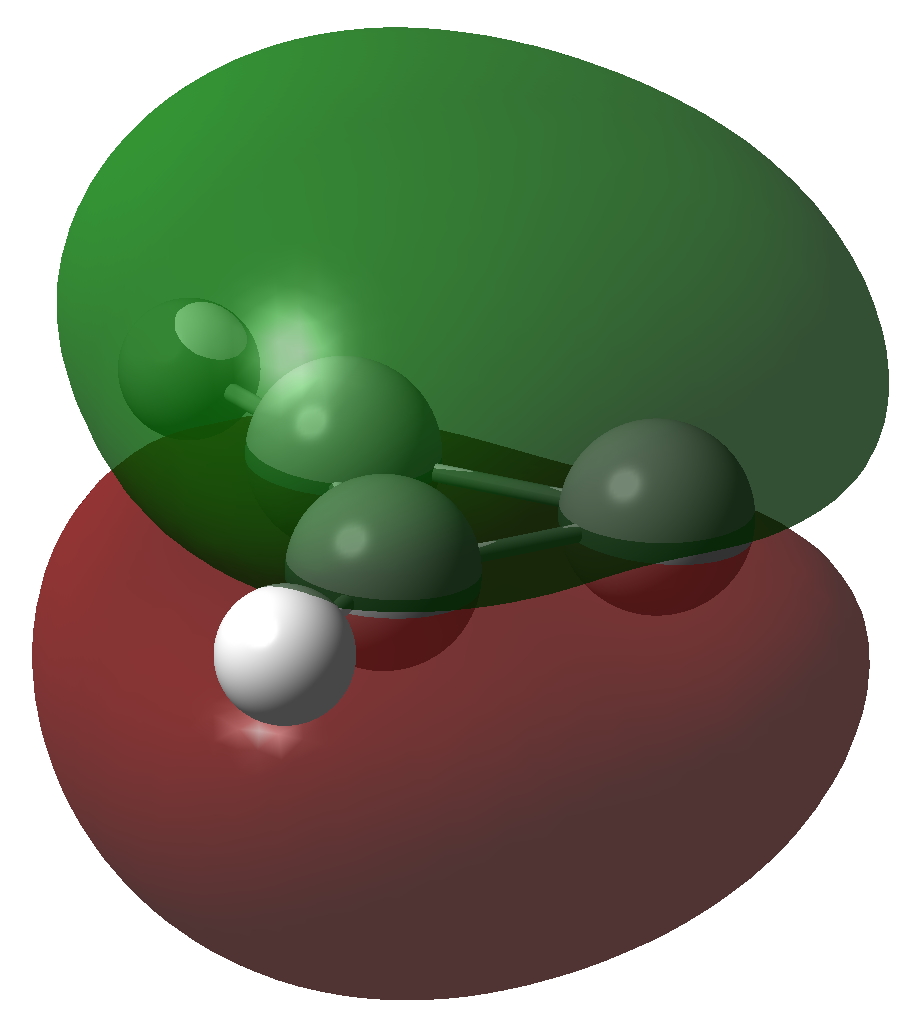 Click to view 3D model of NBO 8 |
| Doubly excited anti-aromatic state of Cyclopropenylidene | |
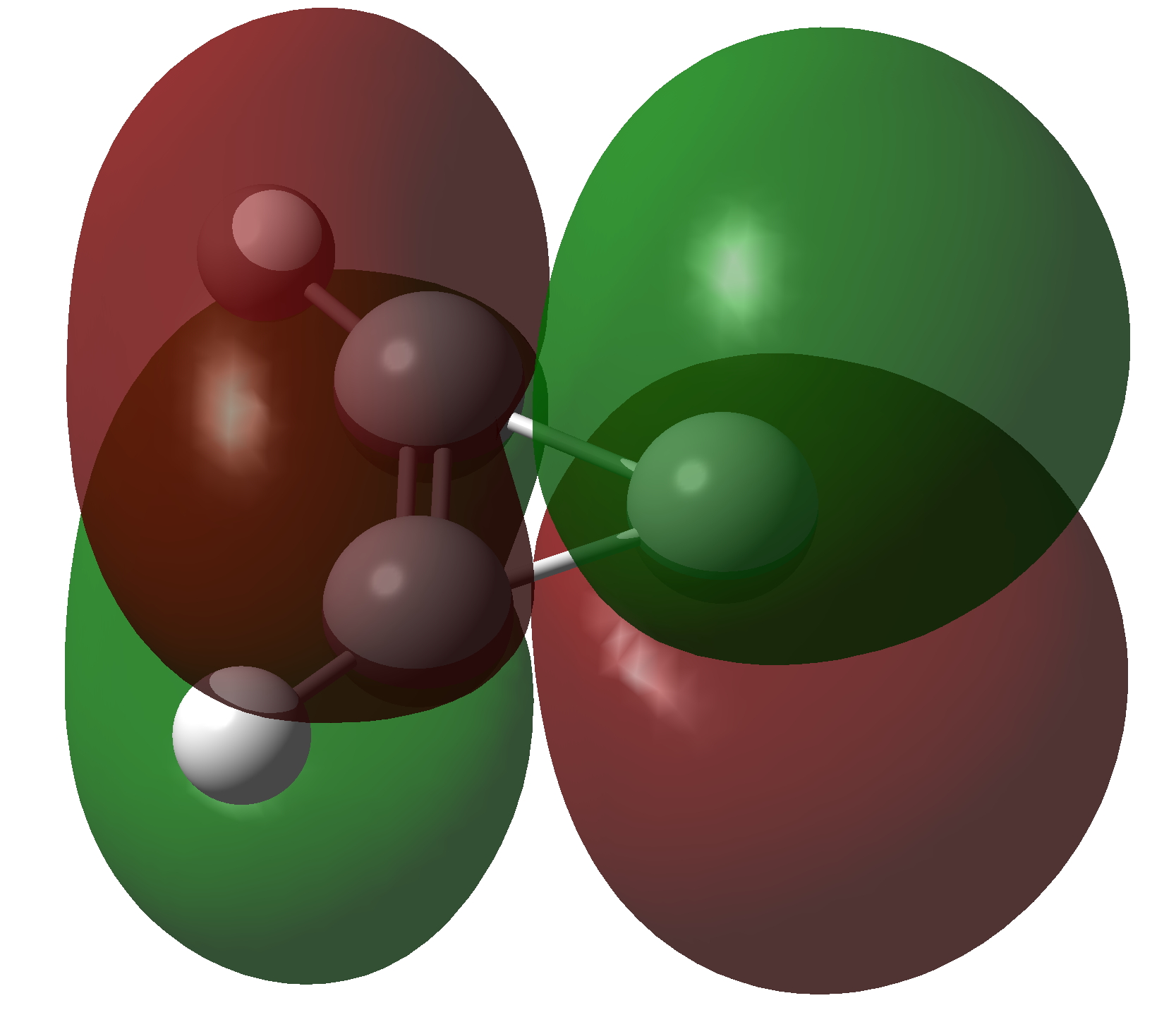 Click to view 3D model of NBO 10 |
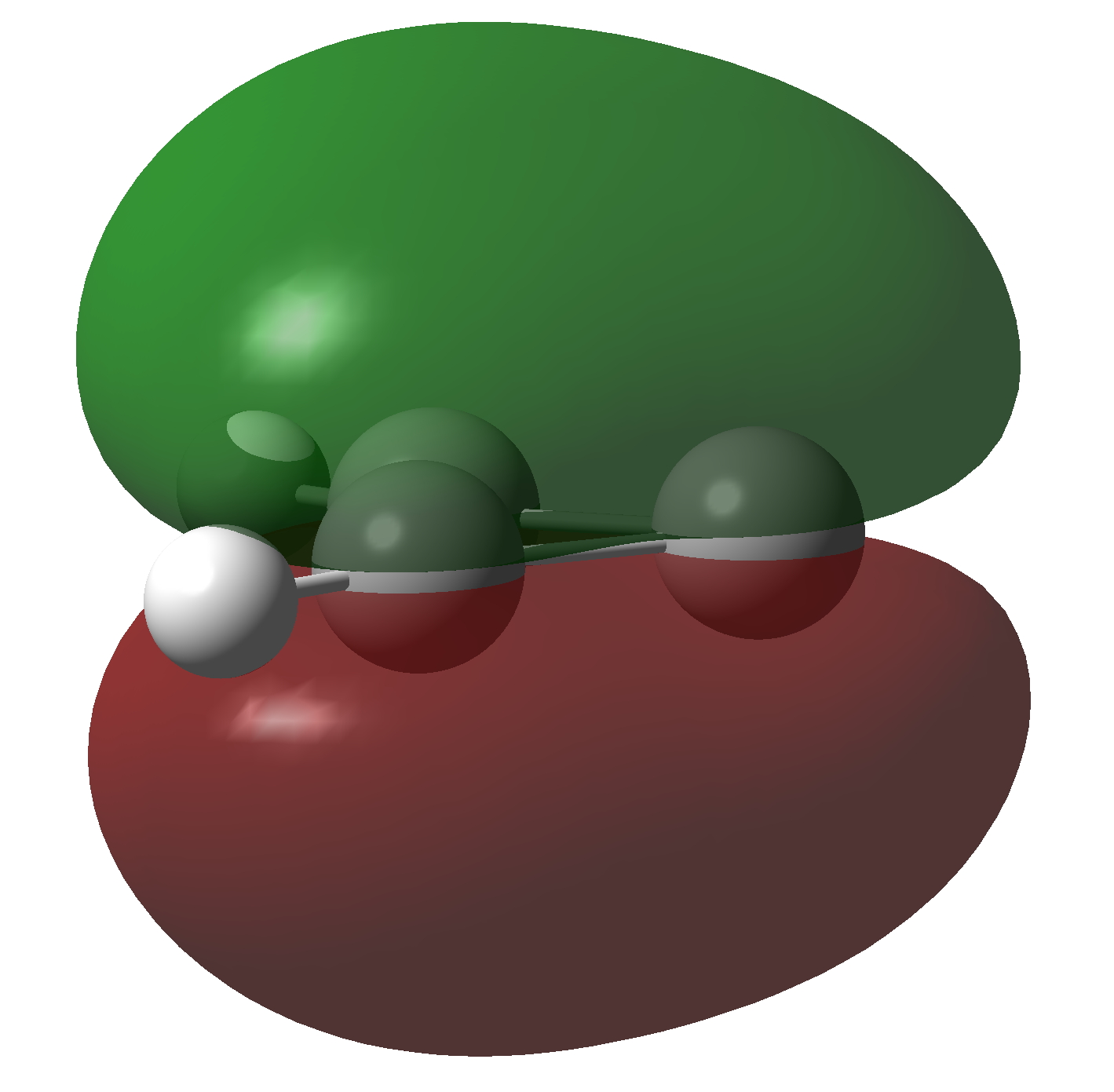 Click to view 3D model of NBO 8 |
It might be a tiny molecule, but to chemists at least it is very interesting in a historical sense at least. Curiously, the astrophysicists describe it as a “complex molecule”!
References
- C.A. Nixon, A.E. Thelen, M.A. Cordiner, Z. Kisiel, S.B. Charnley, E.M. Molter, J. Serigano, P.G.J. Irwin, N.A. Teanby, and Y. Kuan, "Detection of Cyclopropenylidene on Titan with ALMA", The Astronomical Journal, vol. 160, pp. 205, 2020. https://doi.org/10.3847/1538-3881/abb679
What is the dipole moment of this molecule, and do we know if it behaves like a stable carbene?
The dipole moment is 3.5D, which is quite high for such a small molecule. A very quick search of Scifinder does not reveal anything obvious about its potential stability/reactivity.