With the current global lockdown, and students along with everyone else staying at home, I have noticed some old posts of mine are getting more attention than normal. One of these is an analysis I did in 2012 of Robinson’s original curly arrow illustration.[1] That and the fact that I am about to give a lecture on what I call my autobiographical journey discovering them, to our own students here (remotely of course), has prompted me to revisit my original discussion.
Of the two modern representations of nitrosobenzene, the first corresponds to Robinson’s arrows, being an attempt to show, by resonance, that the molecule is o/p directing towards an electrophile. Hence the accumulation of negative charge in the p-position (and other resonance structures can be drawn with it in the o-positions) encouraging electrophilic attack there. The second is the modern version, with the electron flow going in the other direction and hence discouraging electrophilic attack at the o/p positions. All this hinges on the observation that the nitrogen lone pair, involved in the first representation, lies in the plane of the molecule and hence is orthogonal (at 90°) to the π-electrons in the benzene ring and cannot overlap with them. In the modern view, this lone pair plays no part in the resonance.
This can be tested by searching for experimental crystal structures of nitrosobenzenes. I did mention this in the original post, but showed no results. So here is the analysis, in which the plots below analyse the torsion about the phenyl–NO bond. You can see all the examples are either red or blue,‡ which indicates torsions of ~180 or 0°. You can perceive a very nice correlation between the length of the C-N and the N-O bonds. As the latter gets shorter, the former gets longer. This only matches the second resonance shown above and not the Robinson version! Across all known crystal structures of nitrosobenzenes, the balance between these two resonance forms changes, no doubt as a result of substituents on the benzene ring.
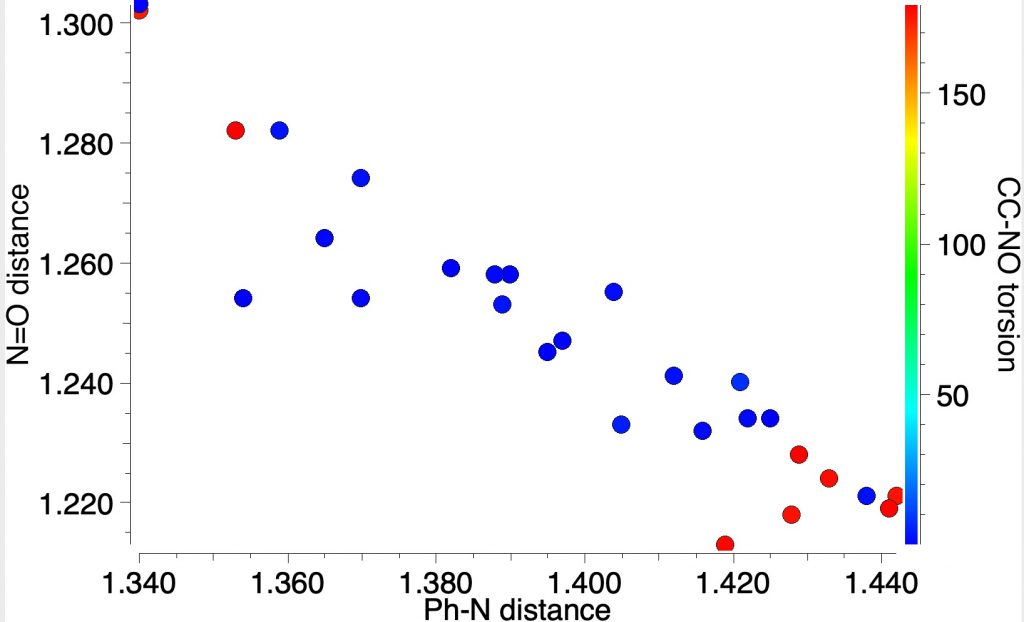 A different plot which now includes the angle at the nitrogen shows very little variation in that angle (113-118°), and certainly not the much larger variation implied by Robinson’s representation. As the N-O bond gets longer, so the angle at the nitrogen opens up a bit, the lone pair on the nitrogen being repulsed by the now three lone pairs on the oxygen anion.
A different plot which now includes the angle at the nitrogen shows very little variation in that angle (113-118°), and certainly not the much larger variation implied by Robinson’s representation. As the N-O bond gets longer, so the angle at the nitrogen opens up a bit, the lone pair on the nitrogen being repulsed by the now three lone pairs on the oxygen anion. 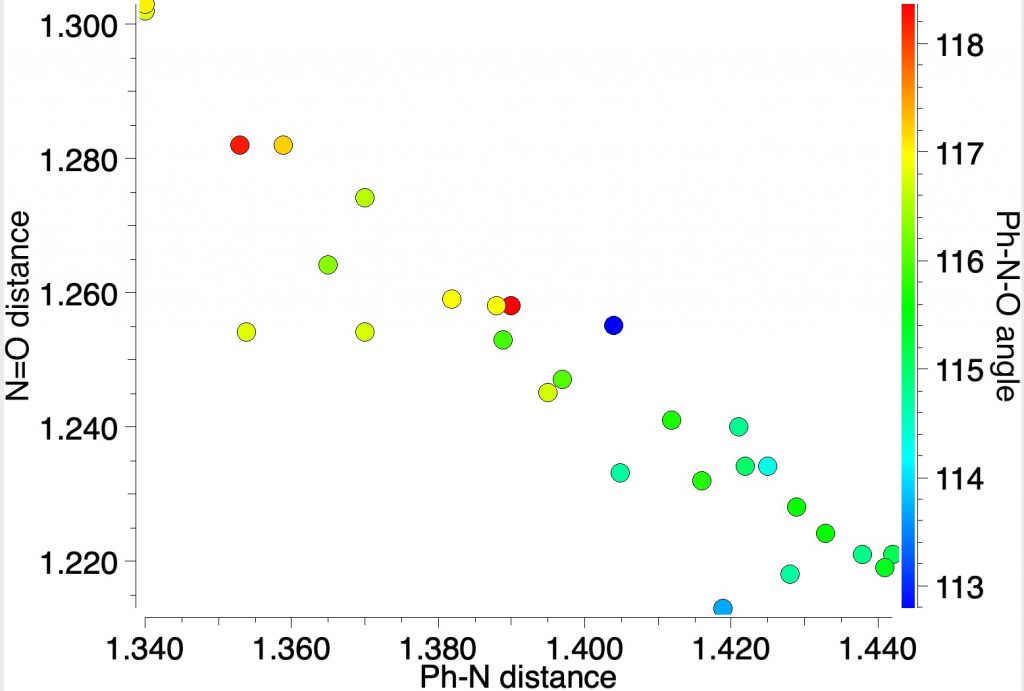
I have noted previously that such crystal structure mining can be used to capture many basic concepts in chemistry.[2] This is a particularly clear one, discriminating between two possible forms of curly arrow. Conversely, it shows how curly arrows can be used to simply rationalise structural variations across a series of compounds.
‡There is one outlier (it does not appear in the plots above, since these are restricted to structures with an R-factor <5%), that shows a linear Ph-N=O system (DOI: 10.5517/cc108dl8) and which may be a Robinson-like valence bond isomer of nitrosobenzene. It will be investigated further!
References
- "Forthcoming events", Journal of the Society of Chemical Industry, vol. 43, pp. 1295-1298, 1924. https://doi.org/10.1002/jctb.5000435208
- H.S. Rzepa, "Discovering More Chemical Concepts from 3D Chemical Information Searches of Crystal Structure Databases", Journal of Chemical Education, vol. 93, pp. 550-554, 2015. https://doi.org/10.1021/acs.jchemed.5b00346
I noted an outlier in the post (DOI: 10.5517/cc108dl8) which shows a linear C-N=O bond, corresponding to the right hand form of Robinson’s resonance for nitrosobenzene. Calculations (10.14469/hpc/7218 show this is around 50 kcal/mol higher in energy than the bent form on the left of Robinson’s structure. One can conclude that this crystal structure is clearly in error.