My previous two posts on the topic of strongest bonds have involved mono and diprotonating N2 and using quantum mechanics to predict the effect this has on the N-N bond via its length and vibrational stetching mode. Such species are very unlikely to be easily observed for verification. But how about a metal M+ instead of H+? It turns out that structures containing the fragment Ru-N≡N-Ru are a small but well studied class of organometallic. Here is a search of the CSD crystal database for this motif.
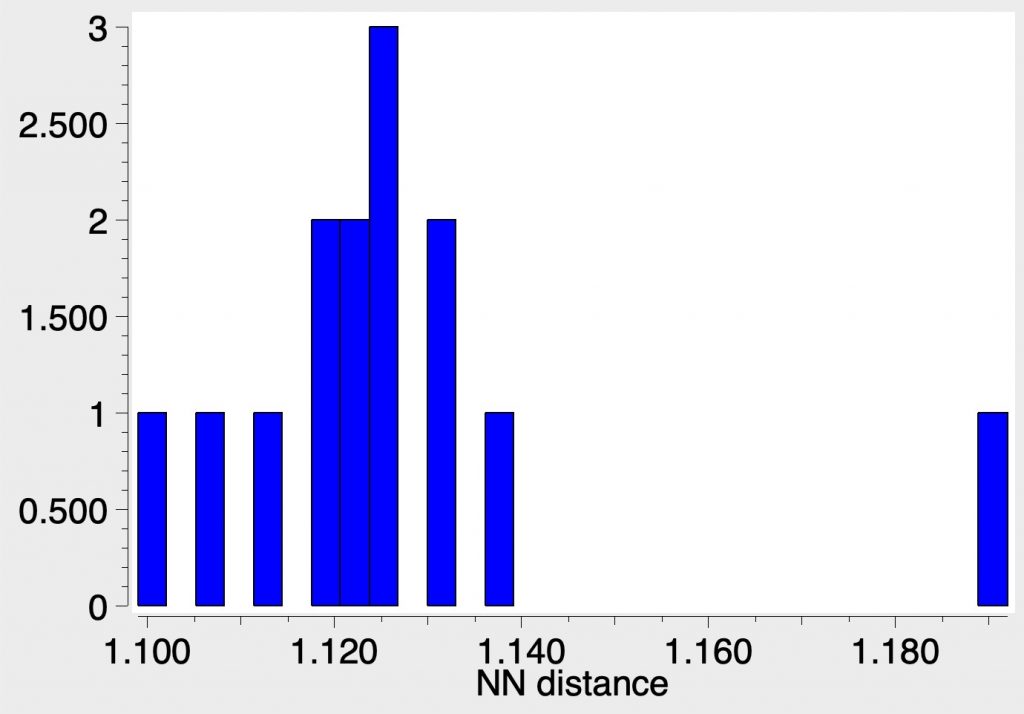
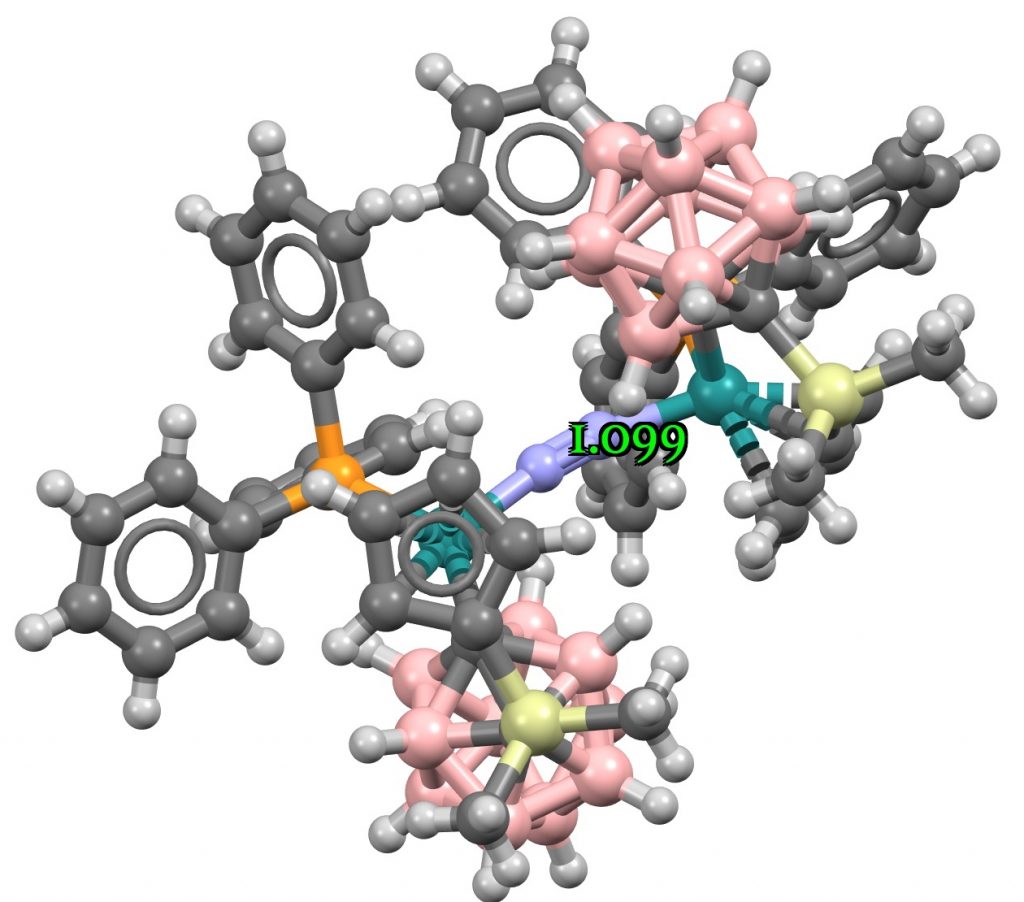
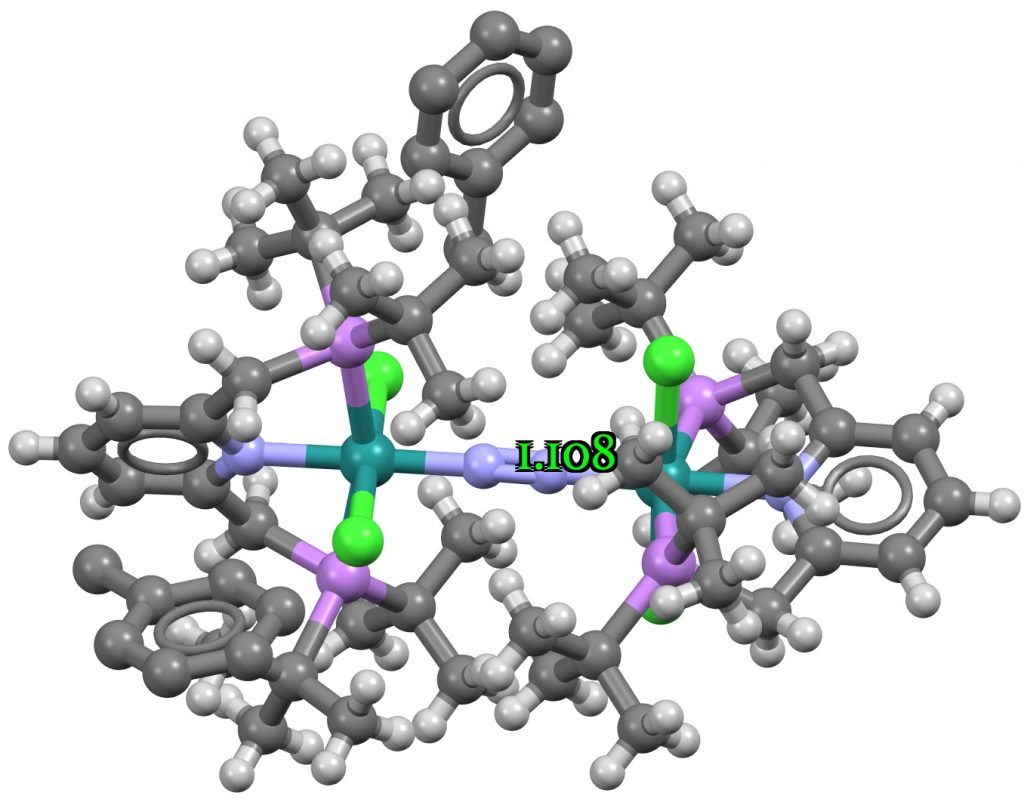
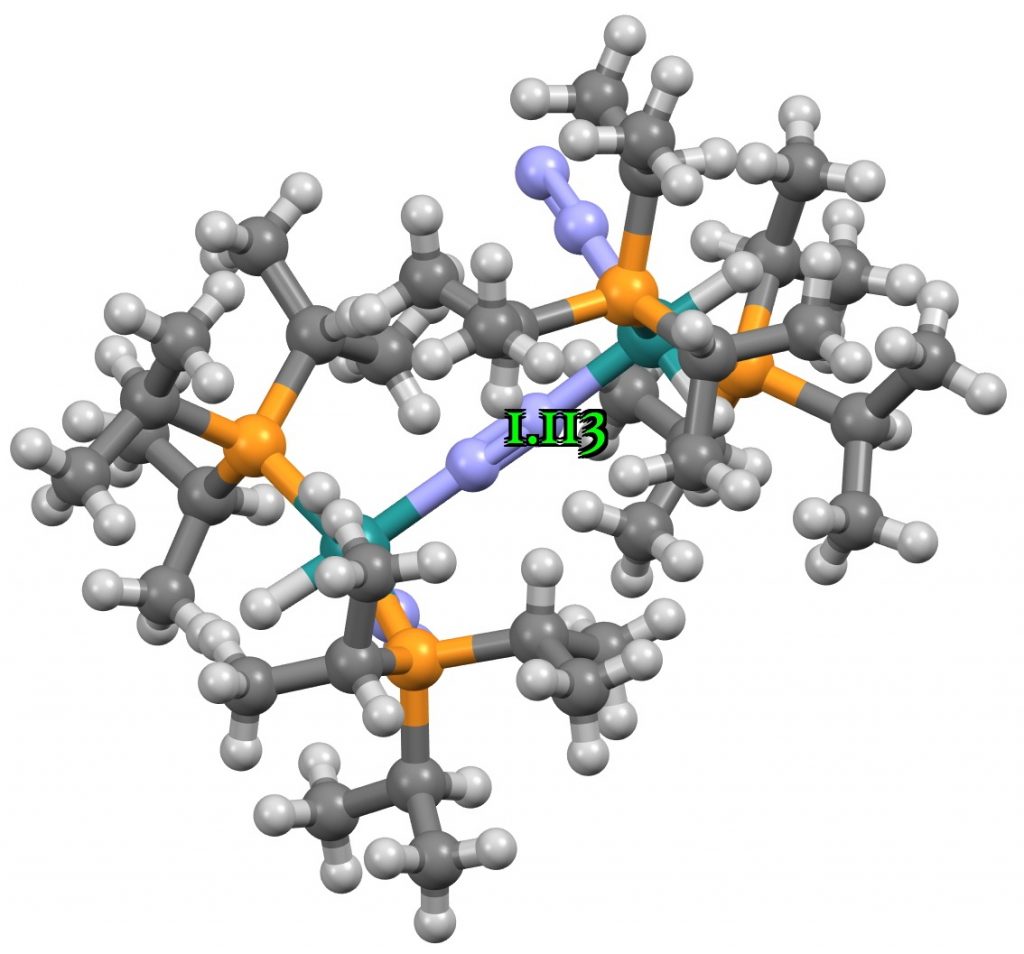
The three examples showing the shortest N-N distances are shown below.[1],[2][3]
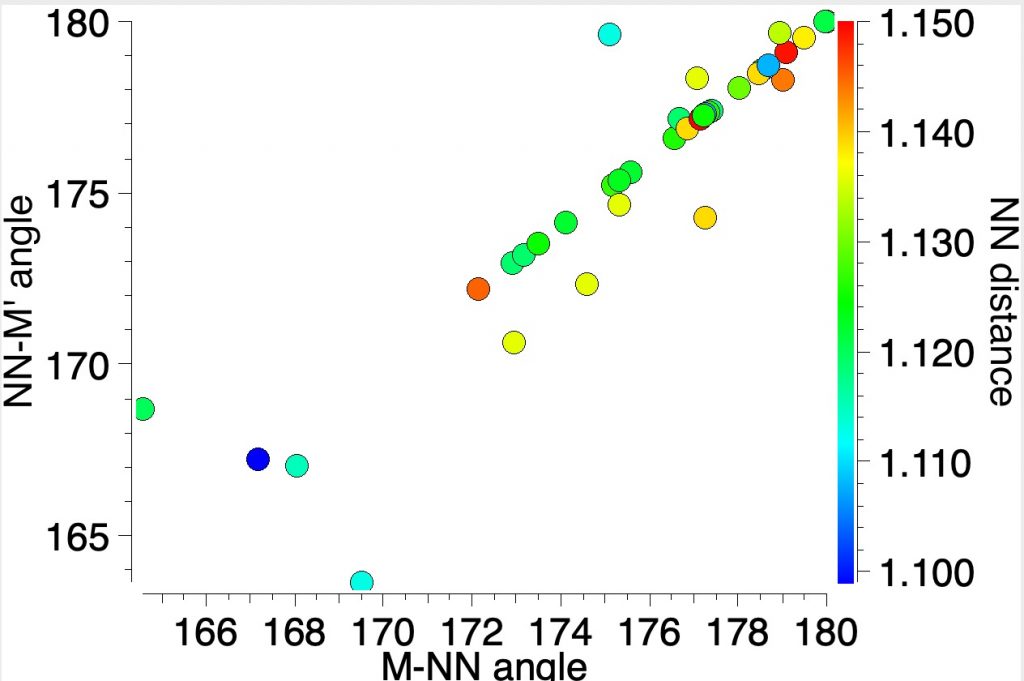
The NN distances for these three examples are in the region of 1.1Å. There are 39 structures in total, for a variety of other transition metals, with a length <1.15Å. The angles subtended at N are close to linear;
For comparison, N2 itself entrained into a crystal structure has the value of ~1.096Å as measured at 80K (R-factor 0.84%)[4]) which is pretty similar to the value computed in the previous post (1.103Å).
So the question to ask is whether any of these organometallic examples have an NN bond at least as strong as that in dinitrogen itself? Only a reliable value for the force constant will give us a clear picture, which however would be non-trivial for such species. But it does suggest that asking whether there could be a real candidate for the strongest bond in the universe other than N2 itself may not be entirely futile.
References
- Y. Sun, H. Chan, and Z. Xie, "Reaction Scope and Mechanism of Sterically Induced Ruthenium-Mediated Intramolecular Coupling of<i>o</i>-Carboranyl with Cyclopentadienyl. Synthesis and Structure of Ruthenium Complexes Incorporating Doubly Linked Cyclopentadienyl−Carboranyl Ligands", Organometallics, vol. 25, pp. 4188-4195, 2006. https://doi.org/10.1021/om0604122
- Y. Tanabe, S. Kuriyama, K. Arashiba, K. Nakajima, and Y. Nishibayashi, "Synthesis and Reactivity of Ruthenium Complexes Bearing Arsenic-Containing Arsenic-Nitrogen-Arsenic-Type Pincer Ligand", Organometallics, vol. 33, pp. 5295-5300, 2014. https://doi.org/10.1021/om5006116
- K. Abdur-Rashid, D.G. Gusev, A.J. Lough, and R.H. Morris, "Synthesis and Characterization of RuH<sub>2</sub>(H<sub>2</sub>)<sub>2</sub>(P<sup>i</sup>Pr<sub>3</sub>)<sub>2</sub> and Related Chemistry. Evidence for a Bis(dihydrogen) Structure", Organometallics, vol. 19, pp. 1652-1660, 2000. https://doi.org/10.1021/om990669i
- C. Dou, W. Kosaka, and H. Miyasaka, "Gate-open-type Sorption in a Zigzag Paddlewheel Ru Dimer Chain Compound with a Phenylenediamine Linker Instructed by a Preliminary Structural Change of Desolvation", Chemistry Letters, vol. 46, pp. 1288-1291, 2017. https://doi.org/10.1246/cl.170509
One might speculate whether London dispersion forces, which have been reported to result in a ultrashort H…H distance of ~1.57Å in a triphenyl methane derivative (DOI: 10.1021/jacs.7b01879 and 10.5517/ccdc.csd.cc1nc1bd) might also be brought to bear on this NN problem. Another example is hexakis(3,5-di-t-butylphenyl)ethane (DOI: 10.1021/ja00286a053) which effectively induces the formation of a central C-C bond in an otherwise unfavourable sitation. Perhaps e.g. the ligands on the metal could be designed to induce such attraction and the NN bond might be prevailed upon to contract further?