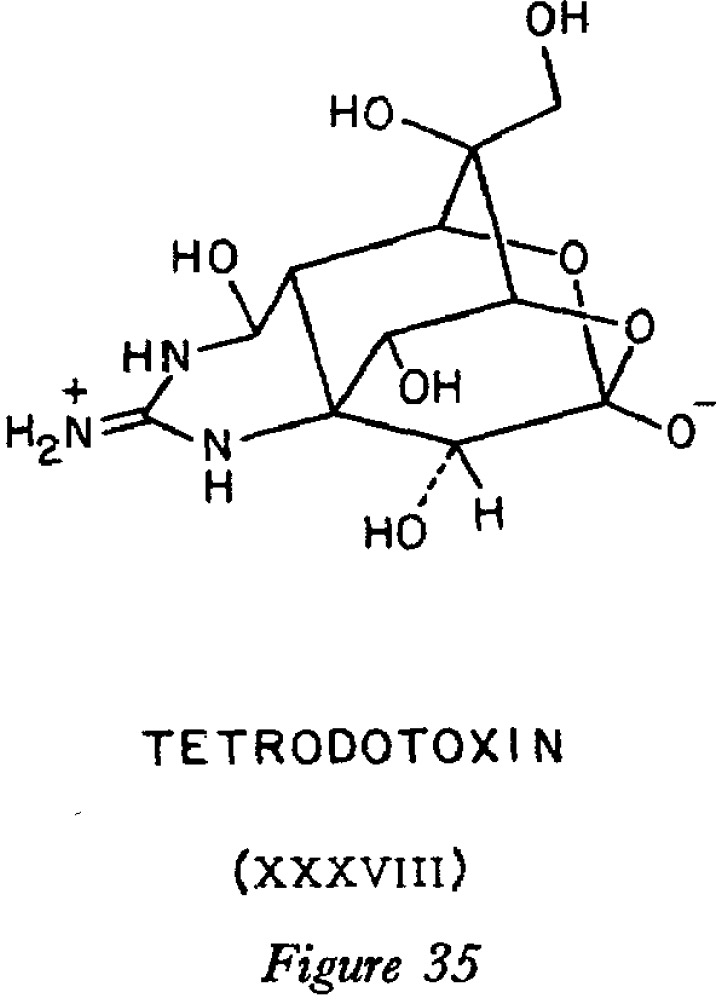
The notorious neurotoxin Tetrodotoxin is often chemically represented as a zwitterion, shown below as 1. This idea seems to originate from a famous article written in 1964 by the legendary organic chemist, Robert Burns Woodward.[1] This structure has propagated on to Wikipedia and is found in many other sources.
With the elegance and the unique style that is typical Woodward, his article is a tour de force because of the way in which he deploys a large armoury of spectroscopic (X-ray crystal,† NMR, IR) as well as physicochemical (pKa) tools to infer this structure; an approach that has been subsequently widely emulated. The article a well worth a read for the elegant logic that slowly builds to a climax on page 73 (sic!) of the article, when he unveils his final structure (XXXVIII, or 38). The lecture(s) from which the article is apparently derived must have been one hell of an occasion.‡
One technique not available in 1964 to Woodward was quantitative quantum calculation of the molecular free energies. This property is now routinely computable, but is still only rarely used for structural problems even today. Here I add this property to Woodward’s collection (FAIR data DOI: 10.14469/hpc/6278, method=ωB97XD/Def2-TZVPP, SCRF=chloroform).
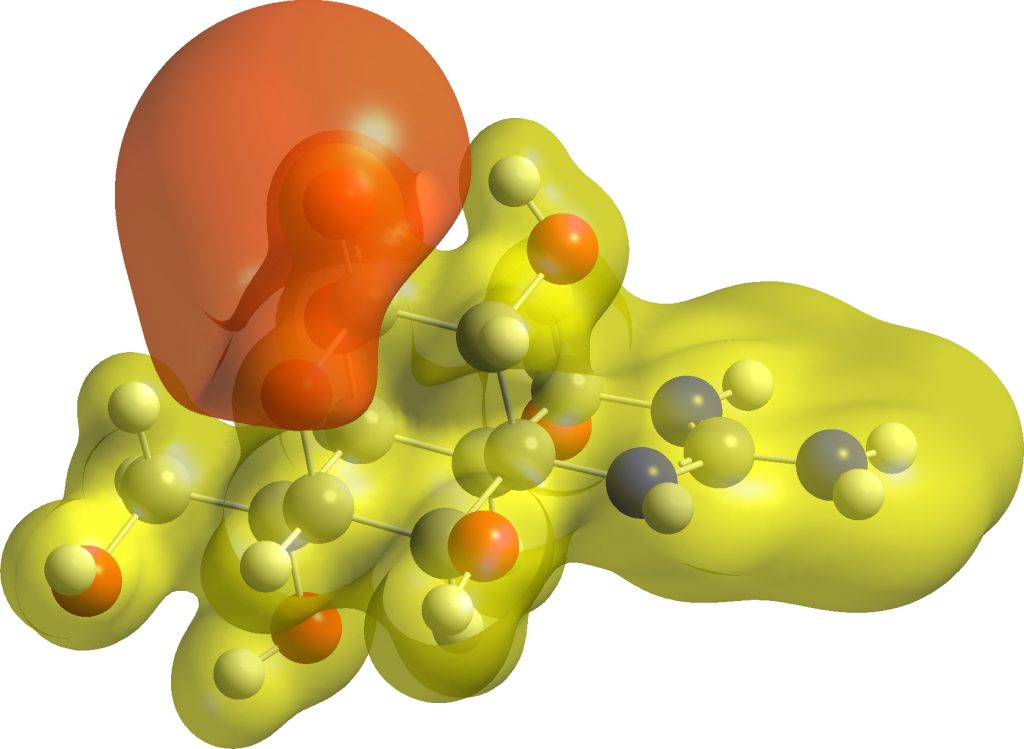
My initial hypothesis was based on the observation of a rather large separation of the charges in the zwitterion. The surface below is the computed molecular electrostatic potential (value 0.1 au), which indeed shows large charge separation. The computed dipole moment is 18D.
Charges by and large do not much like being separated, even though of course the actual separation is often misleadingly indicated by simple Lewis structures such as the above. So I started by moving one proton to produce 2 to see if that improved the free energy, by reducing charge separation. The dipole moment was indeed smaller at 14.6D (although the energy was not lower).
I then included three neutral forms (3–5) where the nominal charge separation is eliminated entirely.
| Structure | Diagram |
ΔΔG kcal/mol |
Dipole moment |
|---|---|---|---|
| 1 | +10.4 | 18.0 | |
| 2 | +21.1 | 14.6 | |
| 3 | 0.0 | 3.9 | |
| 4 | +1.8 | 6.0 | |
| 5 | +4.2 | 6.5 |
This computed model suggests 3 is the predominant species present (in chloroform solutions), although of course all the species are in a prototropic equilibrium. 3 also happens to have the smallest calculated dipole moment. Species 5 was also a front-runner in 1964[2] (as 1c in that article). Certainly the zwitterion 1 as suggested by Woodward, with its large degree of charge separation, is unlikely to feature and 5 is also less likely to be the dominant species.
The computed free energy for such molecules takes < 1 day of elapsed time to produce, and so I here argue that this should be a mandatory reported property for such structural problems.
†The crystal structure is recorded[3] for the protonated species, as the HCl or HBr salt. These of course do not indicate where the protons are for the deprotonated neutral base. ‡I only ever attended one lecture by Woodward. It lasted the typical two hours and was indeed hugely memorable in several regards. I also note the parsimony of stereochemical notation (just one dashed bond), presumably on the grounds that omitting such notation does not actually result in ambiguity. Disambiguation does depend of course on perceived “hidden line” removal.
References
- R.B. Woodward, "The structure of tetrodotoxin", Pure and Applied Chemistry, vol. 9, pp. 49-74, 1964. https://doi.org/10.1351/pac196409010049
- T. Goto, Y. Kishi, S. Takahashi, and Y. Hirata, "Further studies on the structure of tetrodotoxin", Tetrahedron Letters, vol. 5, pp. 779-786, 1964. https://doi.org/10.1016/0040-4039(64)83035-5
- A. Furusaki, Y. Tomiie, and I. Nitta, "The Crystal and Molecular Structure of Tetrodotoxin Hydrobromide", Bulletin of the Chemical Society of Japan, vol. 43, pp. 3332-3341, 1970. https://doi.org/10.1246/bcsj.43.3332
Thank you for this fascinating post, Woodward’s paper is indeed a classic and I thoroughly enjoyed reading it. One small point – Fig XXXVIII is on page 73 of the journal, not the article. The article starts on journal page 50, so the conclusion is on article page 24 – still an impressive length by modern standards.
I also liked reading this paper as it is a good example of structure elucidation with mostly classical means (they had an 1H NMR at 60 MHz, though).
Interestingly, Kishi, Goto et al (Tetrahedron Lett. 1963, 2115; doi:10.1016/S0040-4039(01)90980-3) had considered a neutral form of the currently assumed structure (structure 5 above) even before Woodward.
Those authors wrote: “The ortho-ester type formulas, (Ib) and (Ic), can also be eliminated since they, especially (Ic), can not account for the low pKa value (8.3) …”
It is interesting to note that the pKa values were some of the key arguments in favor of the zwitterionic structure!
Also Woodward based his conclusions on the pKa and explicitely wrote: “The observed pKa 8.5 is too low for a guanidinium system”, and later concluded that: “The free bases are Zwitterions!” (page 67).
@Henry: would your calculation give different results if tetrodotoxin were treated as a solution in an electrolyte (e.g., sea-water), where both alkoxide and guanidinium subunits were allowed to form ion-pairs with Na+ and Cl-?
Clarification to the above post:
• Kishi/Goto rejected the orthoester type structure in favor of an “anti-Bredt” lactam.
• It was Woodward who used the pKa as key supporting evidence in favor of the orthoester Zwitterion, which was not considered by Kishi.
To take up Lukas’ last point about the ionic environment surrounding the zwitterion (rather like e.g. a salt bridge stabilizing ionic amino acids), that is well worth investigating. Even less ionic interactions such as hydrogen bonds may have an impact. I will shortly report on the effect of the latter (using both CHCl3 and H2O) on the relative energy of the zwitterion.