In 2012, I wrote a story of the first ever reaction curly arrows, attributed to Robert Robinson in 1924. At the time there was a great rivalry between him and another UK chemist, Christopher Ingold, with the latter also asserting his claim for their use. As part of the move to White City a lot of bookshelves were cleared out from the old buildings in South Kensington, with the result that yesterday a colleague brought me a slim volume they had found entitled The Journal of the Imperial College Chemical Society (Volume 6).‡
This journal is a record of lectures given to the chemistry department by visiting speakers, this one dating from 1926, about two years after the article by Robinson noted above.
There are a number of points of interest.
- Early on, Ingold introduces the topic of atoms in combination. Lewis (who is acknowledged to have introduced this concept in 1916) is mentioned in parentheses, if not actually in passing, as generalizing (Lewis) from this case, … As was the practice at the time, referencing one’s sources was not always common, and you do not here get an actual citation for Lewis!
- Next comes the topic changes in molecular structure (which could be a synonym for reactions) and here you get this diagram
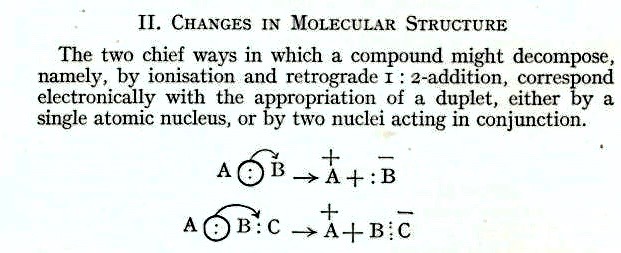 A modern version is shown below, scarcely different!
A modern version is shown below, scarcely different! - Whilst the first example has examples such as SN1 ionizations, the second is perhaps not as common as might be imagined. It would only work if atom C (assuming it to be carbon) was e.g. a carbene (with six valence electrons) converting to a vinyl carbanion (with eight). Although we may speculate that Ingold thought that the second example might relate to common reactions, in the event both curly arrows are still entirely valid by modern standards. There is no acknowledgement of Robinson’s 1924 effort.
- Ingold goes on to discuss substitution patterns in benzene derivatives, and the o/p or m-directing abilities of substituents. He concludes that the Dewar formula for benzene is the most satisfactory vehicle for expressing the theory that electrical disturbances readily reach the o- and p-position, whilst only a small second order effect can reach the m-position. Here I think we can conclude that this approach has not survived into modern thinking. Robinson in his 1924 arrows had of course striven to explain the apparent propensity of nitrosobenzene towards electrophilic substitution in the p-position. Henry Armstrong some thirty years earlier in 1887[1] had arguably already made a pretty decent start, without requiring the use of Dewar benzene.
I suspect those who have dug through the historical archives to cast light on the Robinson/Ingold rivalry may not have appreciated that the Journal of the Imperial College Chemical Society might have been an interesting source!
‡There were nine volumes produced during 1921-1930. It then morphed into The Scientific Journal of the Royal College of Science which continued for an unknown number of years.
References
- H.E. Armstrong, "XXVIII.—An explanation of the laws which govern substitution in the case of benzenoid compounds", J. Chem. Soc., Trans., vol. 51, pp. 258-268, 1887. https://doi.org/10.1039/ct8875100258
Tags: arrow pushing, chemical reaction, Chemical Society, chemist, Chemistry, Christopher Ingold, Christopher Kelk Ingold, College of Science, Country: United Kingdom, Fellows of the Royal Society, Henry Armstrong, Imperial College Chemical Society, Imperial College London, Ingold, Knights Bachelor, Person Career, Robert Robinson, Royal College of Science, The Scientific Journal
