It was about a year ago that I came across a profusion of colour in my local Park. Although colour in fact was the topic that sparked my interest in chemistry many years ago (the fantastic reds produced by diazocoupling reactions), I had never really tracked down the origin of colours in many flowers. It is of course a vast field. Here I take a look at just one class of molecule responsible for many flower colours, anthocyanidin, this being the sugar-free counterpart of the anthocyanins found in nature.
These vary widely in the substituent around the aromatic rings, but here I take a look at just three differing substitutions. Thus pelargonidin has just one OH on ring C (R1‘, R3‘=H, see crystal structure[1]), cyanidin has two (R5‘=H, see crystal structure[2]) and is found in red roses, dahlia, peonies etc. Finally delphinidin (no crystal structure available) has three OHs in that region and is found in yes, delphiniums but also grape skins etc. Below is a colour table that allows one to relate the electronic transitions in a molecule to the observed colour, which of course is due to removal (absorption) of wavelength of light leaving us to see all the remaining wavelengths.
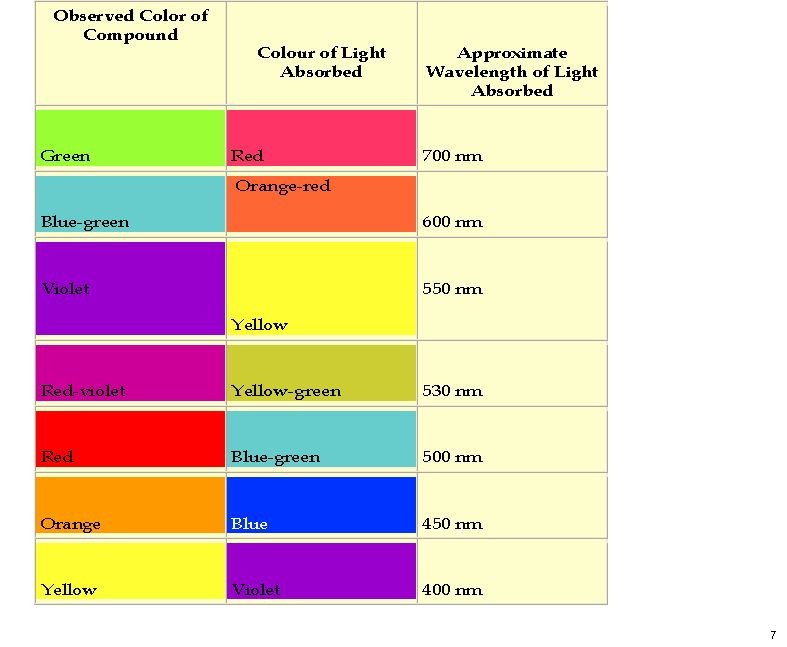
Next I show the computed UV/visible spectra of these three species (ωB97XD/6-311G(d,p)/SCRF=water)‡. Click on any image to se a 3D model of the molecule.
Note how in the visible region, all have a very simple (monochromatic) single electronic transition comprising mostly the HOMO→LUMO excitation.
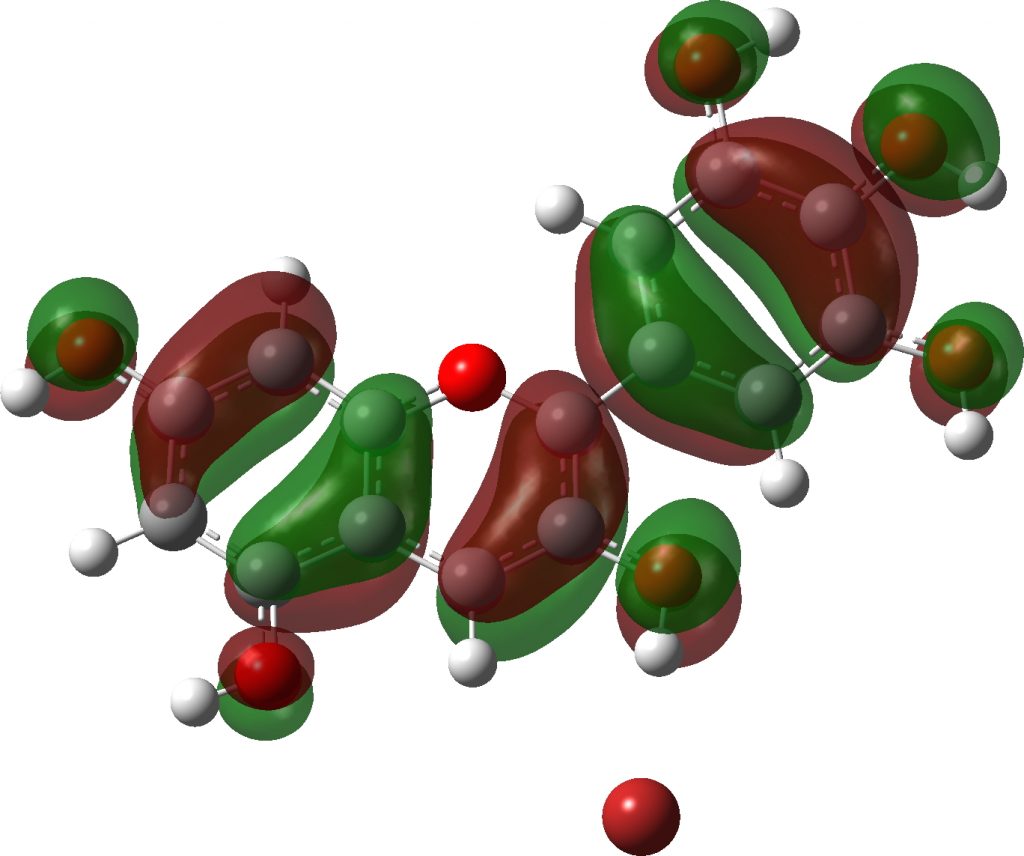
Click to view 3D model of the HOMO
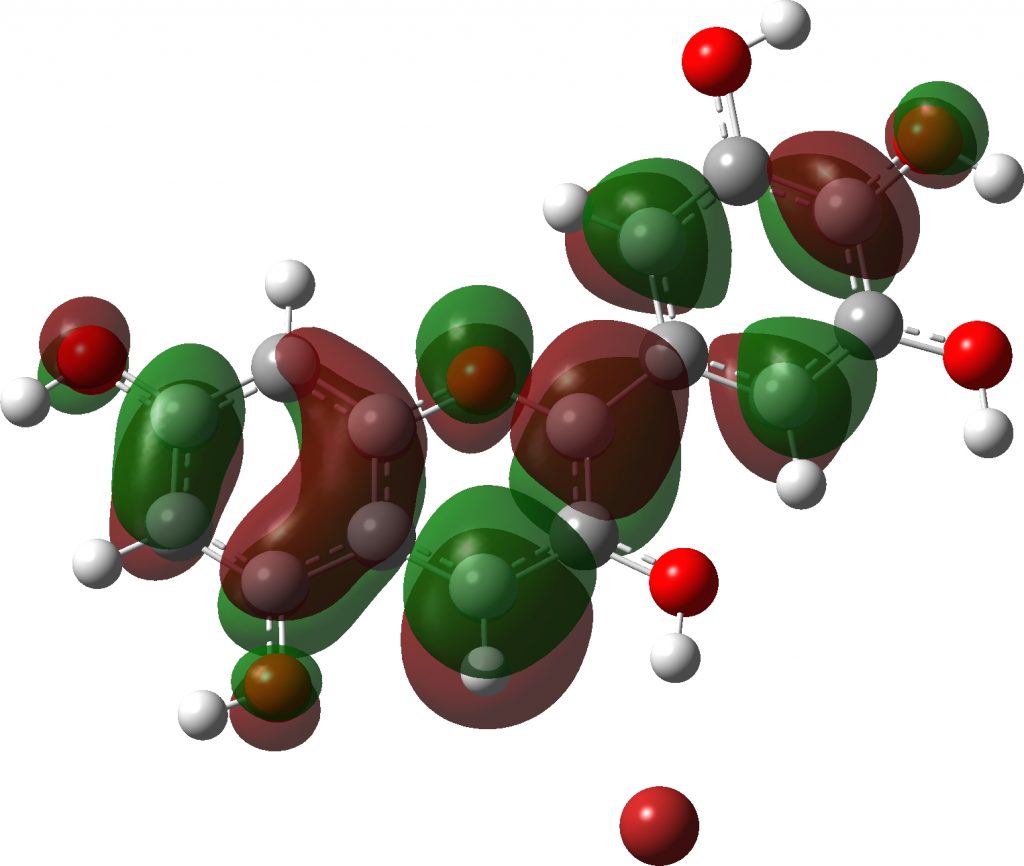
Click to view 3D model of the LUMO
Now, λmax can be predicted quite poorly using most DFT methods, but the trends should be better predicted. Thus the change induced by adding two hydroxy groups is ~7nm, which is in effect how the colour seen in a flower can be tuned to display different shades.
Next, pH. Using delphinidin, under basic conditions one can remove a proton from the cationic species to produce a neutral quinone. In fact, any one of five OH groups could have its proton removed and so it is of some interest to compare the relative energies of the five isomers so produced.
| Position proton removed | Relative ΔG298, kcal/mol |
|---|---|
| 4′ | 0.0 |
| 5 | 3.8 |
| 7 | 4.7 |
| 3′ | 11.8 |
| 5′ | 22.2 |
In fact, one species only would have the major Boltzmann population (4′) and so we need only look at its UV/Visible predicted spectrum. This is shifted 17nm towards the red, thus producing a blue colour in what remains after it is absorbed. The absorption (ε) also increases significantly. Indeed the very striking colour of blue delphiniums (one of my favourite flowers) must be produced by such pH control in the plant. Given the presence of delphinidin in many grape skins, the next time I drink a glass of red wine, I will see if it turns blue upon adding some NaOH!
‡FAIR data doi: 10.14469/hpc/4473
References
- N. Saito, and K. Ueno, "The Crystal and Molecular Structure of Pelargonindin Bromide Monohydrate", HETEROCYCLES, vol. 23, pp. 2709, 1985. https://doi.org/10.3987/r-1985-10-2709
- K. Ueno, and N. Saito, "Cyanidin bromide monohydrate (3,5,7,3',4'-pentahydroxyflavylium bromide monohydrate)", Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry, vol. 33, pp. 114-116, 1977. https://doi.org/10.1107/s0567740877002702
Tags: Anthocyanidin, Anthocyanin, Chemistry, Delphinidin, HOMO/LUMO, Major, Molecular electronic transition, Molecule, Nature, PH indicators, Quantum chemistry, spectroscopy, Ultraviolet–visible spectroscopy
One interesting thing I noticed when reading your post is that grapes, roses, delphiniums, peonies and dahlias are all essentially unrelated – the lowest common clade is the Eudicots (https://en.wikipedia.org/wiki/Eudicots). So did anthocyanidin evolve very early and have been preserved in all these species, or is it a case of convergent evolution because the compounds are easy to make from the precursors available to plants?
I was sure there must be plenty of videos of people adding base (probably NaHSO4) to red wine, but I couldn’t find any quickly. I did find this one on naturally blue wine, although with no information on the chemistry: https://www.youtube.com/watch?v=3Im-VlLgmiI.
Thanks for these insightful remarks!
Yes, I suspect anthocyanins are on an early metabolic pathway common to many flowers.
Regarding blue wines, it would be great to know the pH of this unusual one. Most wines are acidic (pH 4-5 or so), and I suspect to be blue, it would have to be > pH 7.
Making wine from big DEEP purple plums, almost black, I raised the pH using NaHCO3 and where the solution entered the wine there was a transient shift to a much less intense blue. Stopping fermentation with Na2S2O5, which reacts to NaHCO3 + hydrated SO2, produced a beautiful ruby red at the point of mixing. But I’ve no idea what the pigment(s) are in plum skins.
Does this also work for other flowers?