In the previous post, I noted the crystallographic detection of an unusually short non-bonded H…H contact of ~1.5Å, some 0.9Å shorter than twice the van der Waals radius of hydrogen (1.2Å, although some sources quote 1.1Å which would make the contraction ~0.7Å). This was attributed to dispersion attractions accumulating in the rest of the molecule. I asked myself what the potential might be for other elements to reveal significantly contracted non-bonded distances as a result of dispersive attractions.
Here is a simple search of the CSD (Cambridge structure database, query DOI: 10.14469/hpc/2700) for the monovalent halogens as in C-X. The other constraints are R<0.05, no errors, no disorder, normalised hydrogen positions and a non-bonded intermolecular contacts ≥0.4Å shorter than the sum of the van der Waals radii (a recent compendium of atomic values is available here[1]). I should state at the outset that this sort of search for non-bonded contacts is quite effective at revealing errors in the crystallographic determination, despite the search request that there be none. None, that is, that have been identified; there are many that have not been! So this survey only aims to tease out any broad trends, rather than a specific focus on individual systems.
|
Hits |
Comments |
|---|---|
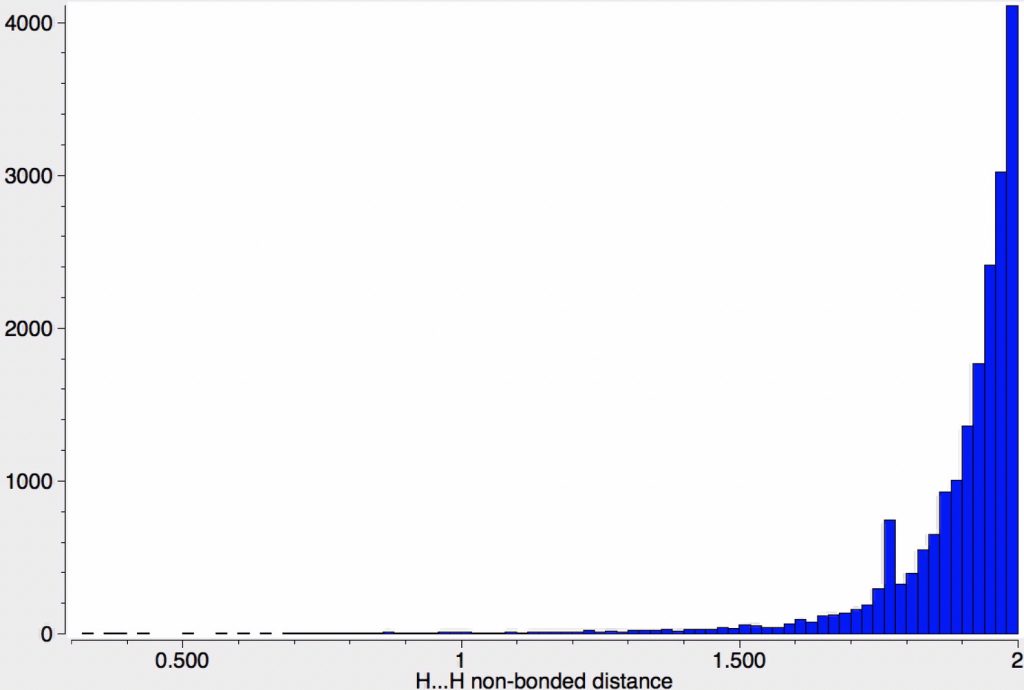
| 15,500 | H…H, vdW sum = 2.4Å; The accepted shortest H…H contacts are around 1.5Å; it is likely the majority of entries (all?) with shorter values are crystallographic errors. There is a curious maximum at ~1.78Å which probably relates to the H…H non-bonded distance in CH2 groups, despite the attempted constraint that the interaction be intermolecular. |
 |
|
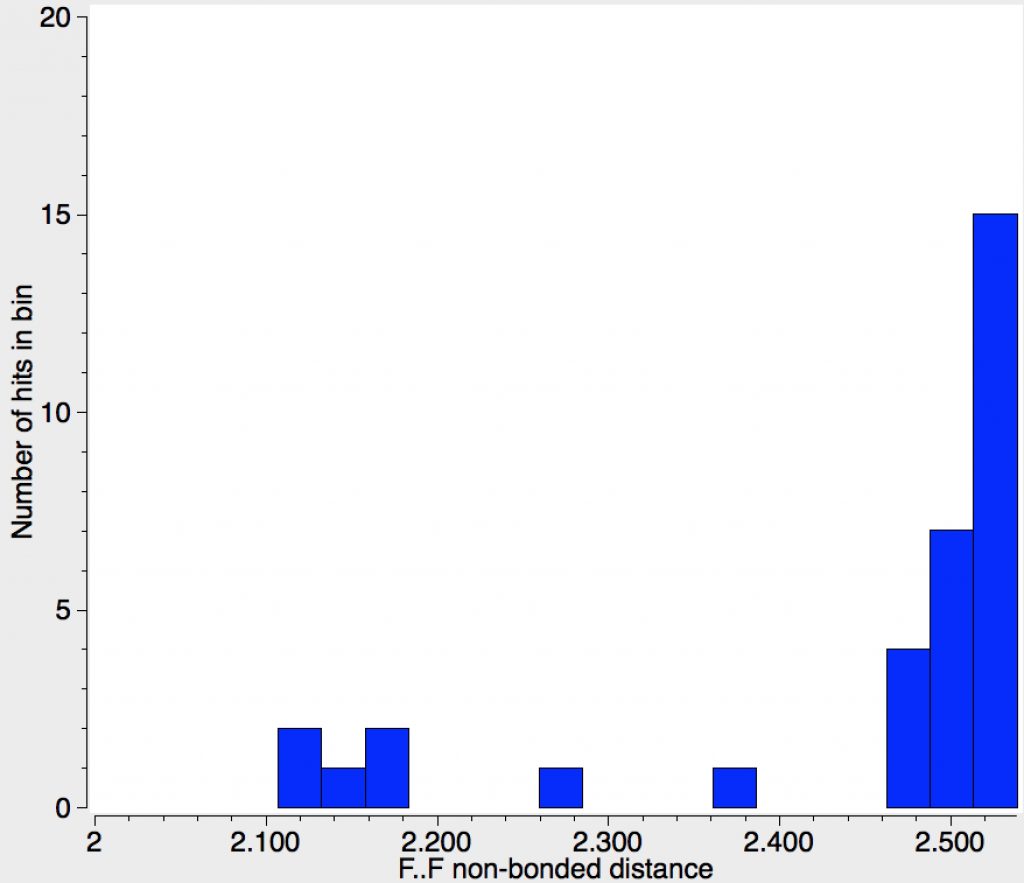
| 31 | F…F, vdW sum = 2.94Å; there are few examples in total and even fewer with the non-bonded distance contracted by ≥0.5Å. Those with ≥0.8Å are probably crystal errors. |
 |
|
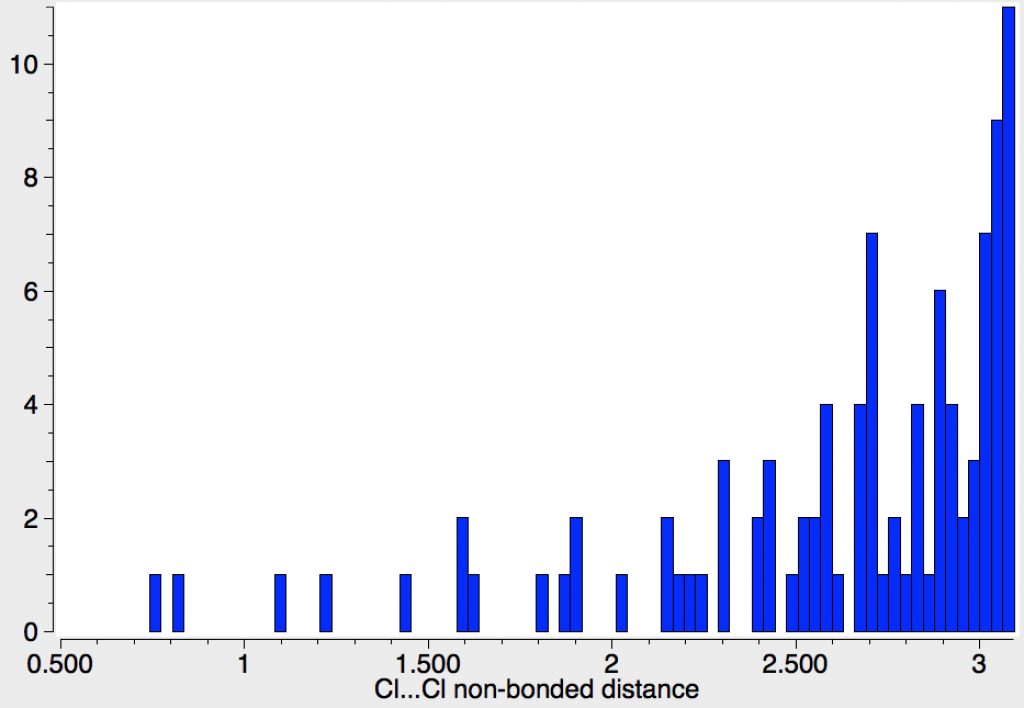
| 70 | Cl…Cl vdW sum =3.5Å. Examples at distances of <2.0Å (a contraction of 1.5Å) are almost certainly errors, even the contractions ~1.0Å are suspect. Does this however indicate Cl is more polarisable than F? |
 |
|
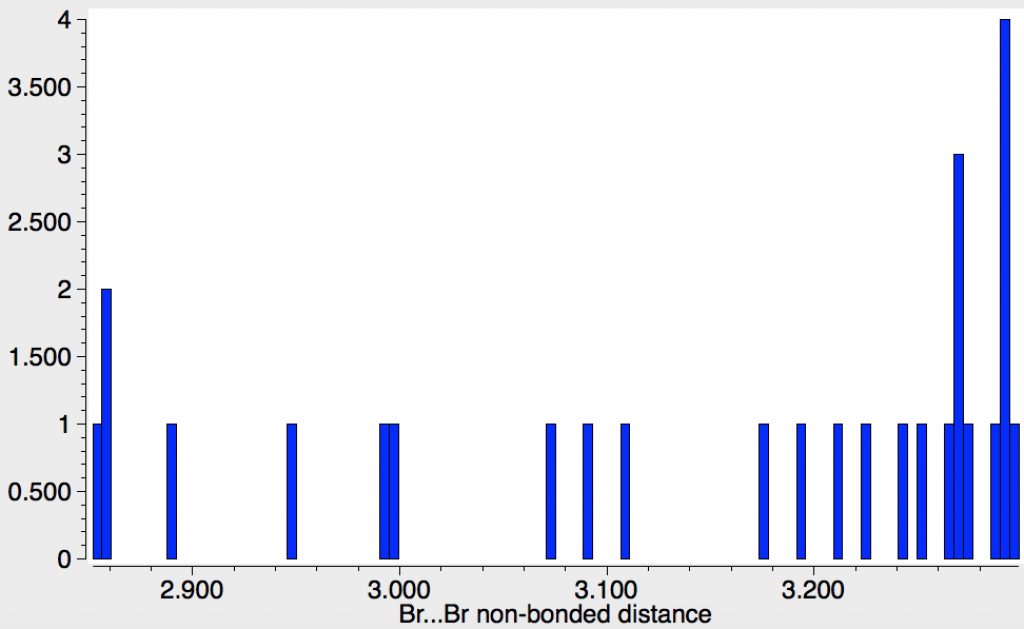
| 26 | Br…Br vdW sum = 3.7Å. Examples contracted by ≥0.4Å are isolated (errors?). |
 |
|
| 10 | I…I vdW sum = 3.96Å. There are no examples contracted by ≥0.5Å; shorter contractions are again errors? |
 |
|
| 1164 | H…F vdW sum = 2.67Å. The separator between real values and probable errors is ~2.1Å, which indicates contractions of 0.6Å are probably real. |
 |
|
| 222 | H…Cl vdW sum = 2.95Å. The separator between real values and probable errors is ~2.4Å; contractions of ~0.5Å are probably real. |
 |
|
| 37 | H…Br vdW sum = 3.05Å. The separator between real values and probable errors is ~2.8Å; contractions of ~0.3Å are probably real. |
 |
|
| 4 | H…I vdW sum = 3.18Å. More data is needed! |
 |
|
We see from these results that the number of short H…H contacts far exceeds those found for the halogen series.‡ Clearly whilst the electron density surrounding H is low, that for the halogens is far higher and hence that electron repulsions are going to be far greater. The effect is attenuated if one partner is H itself, with van der Waals contractions in-between those for X…X and H…H contacts.
This brief survey suggests that H…H distances are by far the best for probing close contacts brought about by dispersion attractions. Perhaps the next element to focus on for such effects might be chlorine rather than fluorine. For example, see DOI: 10.5517/cczjyhx, being Ph3C-Cl…Cl-CPh3 where the Cl…Cl distance is contracted by ~0.3Å. What would happen if the Ph groups were adorned with t-butyl groups to increase the dispersion attractions?
‡ It might of course also mean that the van der Waals radius for H is set too high.
References
- S. Alvarez, "A cartography of the van der Waals territories", Dalton Transactions, vol. 42, pp. 8617, 2013. https://doi.org/10.1039/c3dt50599e
Indeed interesting but is hard to see the distribution of H…H distances below 1.5 Å. Can you prepare a separate figure just between 0-1.5 Å?
Here is an expanded plot, up to 1.5Å H…H contact distance. As I noted before, it is likely most (if not all) are errors.
Thanks. I understand your point and I agree that probably most of them are just errors but even if just one of them to be “real” then that will be a real discovery. The number of molecules do not seem very large based on this plot (I guess around two or three hundred not more) so may be it is worth to check the data more closely to see whether there are any real candidates (Even if something won’t emerge it will fun!). Based on my own experience on the ultrashort H…H contacts, in theory, there is a chance that something real emerges between 1.1-1.5 A. I will be glad to join the effort.
There are 340 entries in the plot above. I deposited the REFCODES into the data repository as a spreadsheet at 10.14469/hpc/2700. I guess its a matter of going through each one individually. I agree, if even just one were to have no discernible errors, that would indeed be interesting!
Let me check the structures from CSD. Indeed, this must be done one by one.
A molecule with stong Cl-Cl intramolecular interactions is hexadecachloro-bis-9,9’fluorenylidene, see X-Ray E. Mollins et al. J. Org. Chem. 2002, 67, 7175. Henry, you may look using your analysis.
We have looked for intramolecular CH3-benzene interaction, see X-Ray in Chem. Ber. 1985, 118, 363-369. Force field MMPI was not so good, because the force field does not consider attractive interactions.
Thanks Dieter. Yes HUTLOX (DOI: 10.1021/jo010979m) shows a Cl-Cl contact of 3.1Å a contraction of ~0.4Å. But this is as you say an intramolecular contact (i.e. it has little choice). Ph3C-Cl…Cl-CPh3 was different in that it was intermolecular.
Following my observation that Ph3C-Cl…Cl-CPh3 exhibits a shortened Cl…Cl distance, I tried adding 3,5-tert-butyl groups to all six phenyl rings to see if this might draw the two halves in and compress the Cl…Cl bond further (DOI: https://doi.org/10.14469/hpc/2701). This distance is predicted as 3.58Å and therefore suggests that the 3,5-tert-butyl groups do not have the right shape to perform this task.