Chemical and engineering news (C&EN) is asking people to vote for their molecule of the year from six highlighted candidates. This reminded me of the history of internet-based “molecules of the moment“. It is thought that the concept originated in December 1995 here at Imperial and in January 1996 at Bristol University by Paul May and we were joined by Karl Harrison at Oxford shortly thereafter. Quite a few more such sites followed this concept, differentiated by their time intervals of weeks, months or years. The genre is well suited for internet display because of plugins or “helpers” such as Rasmol, Chime, Jmol and now JSmol which allow the three dimensions of molecular structures to be explored by the reader. Here I discuss a second candidate from the C&EN list; a ferrocene-based Ferris wheel[1],[2] (DOI for 3D model: 10.5517/CCDC.CSD.CC1JPKYQ) originating from research carried out at Imperial by Tim Albrecht, Nick Long and colleagues.
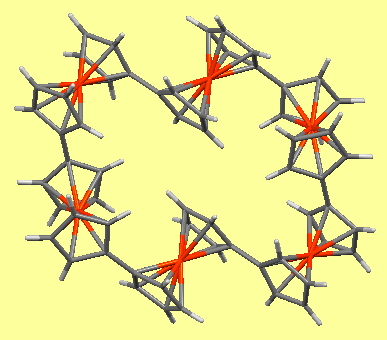
The chemical interest was the redox chemistry of the six metal centres, and the interactions between these centres, expressed more succinctly as “do the iron centres talk to each other?”. The suggestion was that the charges in the molecules originating from oxidation move between ferrocene centres at a rate that is fast compared to the electrochemical timescale. An analogy is drawn to the nanoscale and uniformly charged conductive rings.
I was interested to compare this system with any similar Fe compounds that might also be known in the CSD (Cambridge structure database). Here are some that I found:
- CEFDOG[3] with two cyclic ferrocene units with both neutral Fe and Fe(+) present
- EZEVIO[4], 3D: 10.5517/CC805N2 with Fe and Ge as the metals.
- FULVFE[5] from 1969 with two Fe centres.
- PETTUD and PETVAL[6] with two Fe centres.
- PETVEP and PETVIT[6] with Fe and Zr centres
- URAFUQ and URAGAX (3D: 10.5517/CCDC.CSD.CC1JPKZR), the system shown above.
- VOKXOI[7] with one Fe and one Fe+.
- VOKXUO[7] with one Fe and one Co+.
- WOJDOQ[8], 3D : 10.5517/CC133PGC from 2014 with three Fe units.
- ZECTOQ[9] with one Fe and one Th.
Returning to the communication between ferrocene units, the six-unit ferris wheel noted above has four sets differentiated from the other two in the solid state, although in solution by NMR they are all seen as equalised by exchange. The twist angle between four pairs is ~47° (C-C distance 1.471Å) and for the other two it is ~18° (C-C distance 1.466Å) which allows a fair measure of π-π conjugation to operate between the rings. Contrast this with the smaller WOJDOQ[10], where the torsions between the rings are closer to 80° (C-C distance 1.486Å) thus inhibiting π-π conjugation. It would certainly be interesting to compare e.g. the cyclic voltammetry for these two species to see if electronic communication between the rings is affected by this structural difference.
WOJDOQ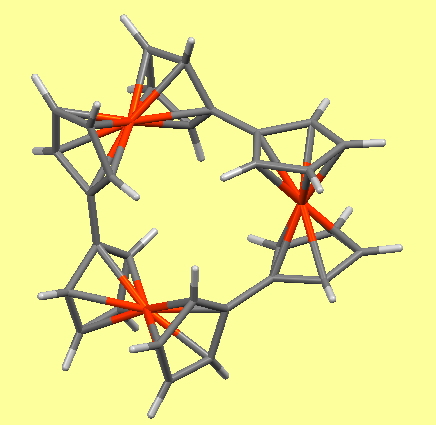
In regard to the D3-symmetric WOJDOQ[10], this is of course chiral and here its chiroptical properties intrigue,‡ along with questions of whether the two enantiomers are configurationally stable at room temperatures. If so, perchance they might be capable of acting as asymmetric catalysts?
Finally I speculate whether these sorts of rings can be constructed as Möbius strips or perhaps even as trefoil knots. It is certainly nice to see new molecules that spark all sorts of interesting new ideas!
‡The calculated optical rotation of WOJDOQ (TPSSh/6-311G(d,p)/SCRF=dichloromethane) is 427° at 800 nm and 1077° at 589 nm (doi: 10.14469/hpc/1971); the VCD (ωB97XD/6-311G(d,p)/SCRF=dcm) is shown below (doi: 10.14469/hpc/1970);
the ECD (doi: 10.14469/hpc/1972 ):
References
- M.S. Inkpen, S. Scheerer, M. Linseis, A.J.P. White, R.F. Winter, T. Albrecht, and N.J. Long, "Oligomeric ferrocene rings", Nature Chemistry, vol. 8, pp. 825-830, 2016. https://doi.org/10.1038/nchem.2553
- Inkpen, Michael S.., Scheerer, Stefan., Linseis, Michael., White, Andrew J.P.., Winter, Rainer F.., Albrecht, Tim., and Long, Nicholas J.., "CCDC 1420914: Experimental Crystal Structure Determination", 2016. https://doi.org/10.5517/ccdc.csd.cc1jpkyq
- M. Hillman, and A. Kvick, "Structural consequences of oxidation of ferrocene derivatives. 1. [0.0]Ferrocenophanium picrate hemihydroquinone", Organometallics, vol. 2, pp. 1780-1785, 1983. https://doi.org/10.1021/om50006a013
- M. Joudat, A. Castel, F. Delpech, P. Rivière, A. Mcheik, H. Gornitzka, S. Massou, and A. Sournia-Saquet, "Synthesis, Structures, and Reactivity of Mono- and Bis(ferrocenyl)-Substituted Group 14 Metallocenes", Organometallics, vol. 23, pp. 3147-3152, 2004. https://doi.org/10.1021/om0400393
- M.R. Churchill, and J. Wormald, "Crystal and molecular structure of bis(fulvalene)diiron", Inorganic Chemistry, vol. 8, pp. 1970-1974, 1969. https://doi.org/10.1021/ic50079a030
- P. Scott, U. Rief, J. Diebold, and H.H. Brintzinger, "ansa-Metallocene derivatives. 28. Homo- and heterobimetallic bis(fulvalene) complexes from bis(cyclopentadienyl)- and bis(indenyl)-substituted ferrocenes", Organometallics, vol. 12, pp. 3094-3101, 1993. https://doi.org/10.1021/om00032a036
- P. Brüggeller, P. Jaitner, and H. Schottenberger, "Kristallographische Gegenüberstellung der Monokationen von Bis(fulvalen)dieisien und Bis(fulvalen) eisen-cobalt mit identischem Gegenion (PF6−)", Journal of Organometallic Chemistry, vol. 417, pp. C53-C58, 1991. https://doi.org/10.1016/0022-328x(91)80206-y
- R. Shekurov, V. Miluykov, O. Kataeva, A. Tufatullin, and O. Sinyashin, "Crystal structure of cyclic tris(ferrocene-1,1′-diyl)", Acta Crystallographica Section E Structure Reports Online, vol. 70, pp. m318-m319, 2014. https://doi.org/10.1107/s1600536814017346
- P. Scott, and P.B. Hitchcock, "Synthesis, structure and electrochemistry of the first fulvalene derivative of an actinide", Journal of Organometallic Chemistry, vol. 497, pp. C1-C3, 1995. https://doi.org/10.1016/0022-328x(95)00108-3
Tags: American Chemical Society, Bristol University, Chemical & Engineering News, Chemistry, Engineering, internet display, Karl Harrison, metal centres, Nick Long, Paul May, Tim Albrecht
The topic above covered cyclic metallocene oligomers containing at least one Fe in the ring. If one releases this constraint to allow any transition metals to be present, one gets 8 systems in total representing the cyclic dimers (two metal atoms). The search query and search results can be found at doi: 10.14469/hpc/1974 and can be summarised;
One with two Co(+), three with two Fe (see above), one with two Ni, one with two MoH (and a supposed Mo-Mo bond linking the two metals), one with Ru and Co(+), and one with Fe and Co(+).
XITGEM (DOI: 10.1246/cl.2001.996) is an interesting variation on the above theme. One cyclopentadienyl anion unit is replaced by a cyclobutenyl di-anionic linkage (bearing not an Fe but a Co) and the linkage is not 1,1′ but 1,2.