To quote from Wikipedia: in chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The most ubiquitous type of carbene of recent times is the one shown below as 1, often referred to as a resonance stabilised or persistent carbene. This type is of interest because of its ability to act as a ligand to an astonishingly wide variety of metals, with many of the resulting complexes being important catalysts. The Wiki page on persistent carbenes shows them throughout in form 1 below, thus reinforcing the belief that they have a valence of two and by implication six (2×2 shared + 2 unshared) electrons in the valence shell of carbon. Here I consider whether this name is really appropriate.
Let us start by counting the electrons in the 2p atomic orbitals on the ring atoms of 1, forming what we call a π-system. There are six; two from the carbons shown connected by a double bond, C=C and a further four from the two nitrogen lone pairs. Now in benzene, we also have six π-electrons in a ring and this molecule is of course famously aromatic due to the diatropic ring current created by the circulation of these six electrons. Moreover, all the C-C bonds are equal in length, ~1.4Å long (although the reasons for this equality are subtle).
So does 1 behave similarly? A ωB97XD/Def2-TZVPP calculation[1] shows the following calculated structure, in which all the bonds are clearly intermediate between single and double. The N-C(“carbene”) length of 1.357Å in particular is much shorter than a C-N single bond (~1.44AÅ), which tends to suggest that the resonance form 2 is a better representation than 1. This form is also pretty similar to pyrrole, itself a well-known hetero-aromatic species.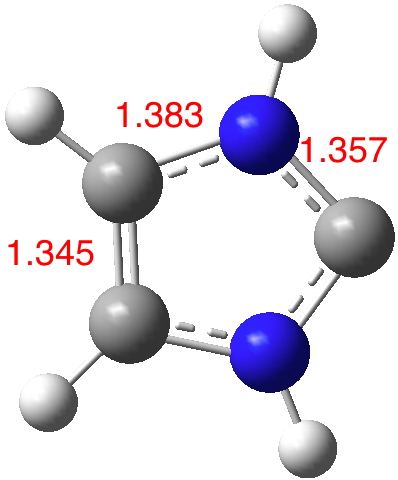
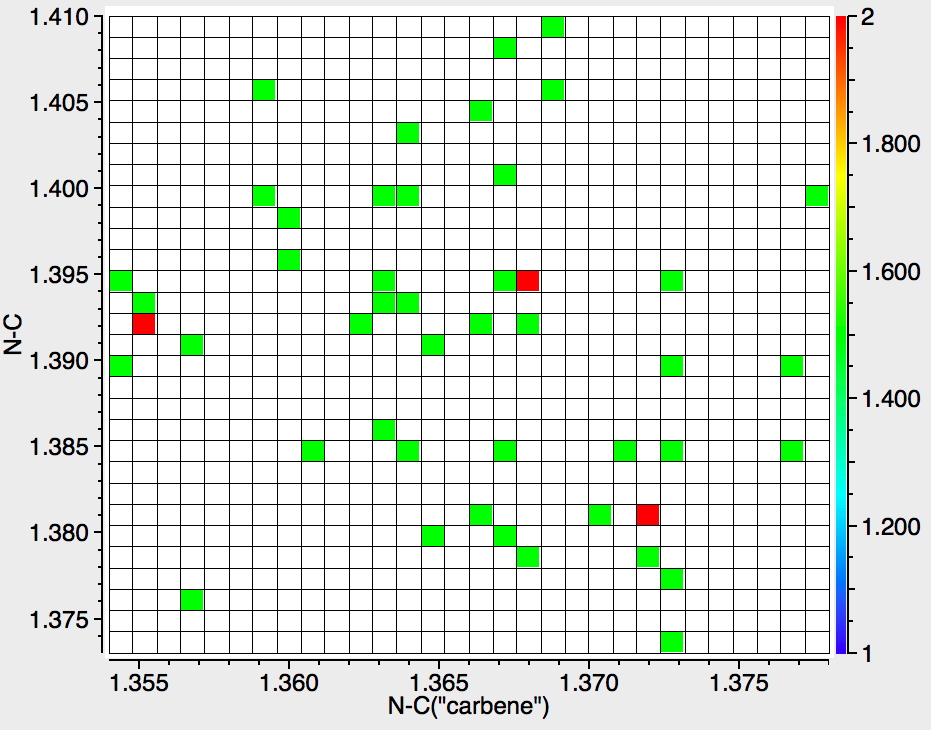
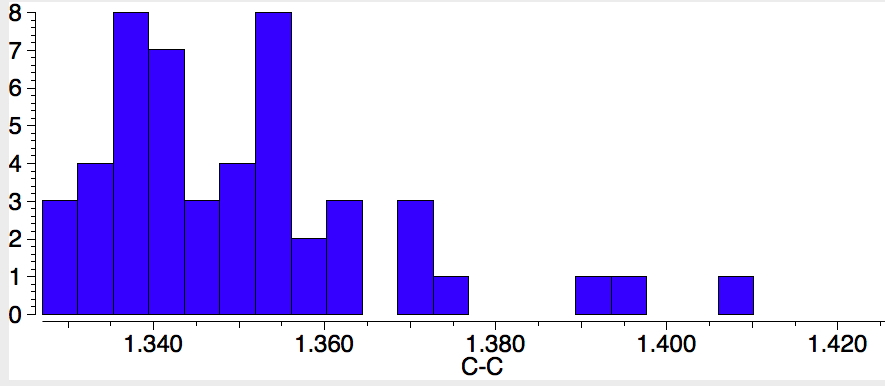
An alternative reality check is crystal structures. There are 42 examples (no errors, no disorder, R < 0.05) in the Cambridge structure database (CSD) and the distribution of C-N bond lengths below is indeed quite similar to the calculation shown above for the unsubstituted parent, with the lhs “hot-spot” almost exactly coincident. The C-C length similarly corresponds.
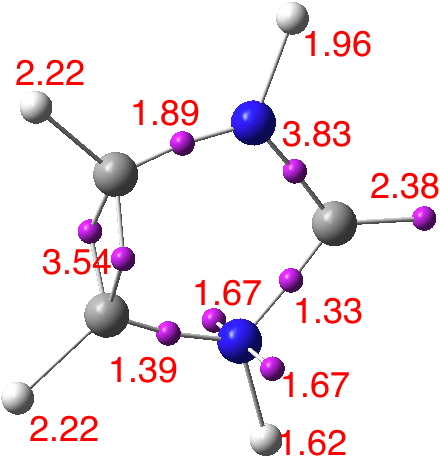
Let us try a technique for explicitly counting electrons, the ELF (electron localisation method), which works directly on a function of the electron density to identify the centroids of localized “basins” containing the integrated density. The three surrounding the “carbene” atom sum to 7.54e (with small seepage also into the carbon 1s core; 2.08e). A “normal” carbon on the C=C bond is 7.65e. The localization below turns out to closely resemble resonance structure 2 above.
Further in-silico experiments can be carried out with species 3 and 4, in which a carbon atom replaces each of the nitrogens. This reduces the total electron count by two and now this poor molecule has a difficult choice to make. Should it be the π-system that sacrifices these two electrons, or could it be the σ-lone-pair found on the two-coordinate carbon? We will let the quantum mechanical solution decide[2] (with a constraint that the molecule be planar). The electrons arrange themselves to resemble the resonance form 4, choosing to retain the six π-electrons and sacrifice the carbene “unshared pair”. The 2-coordinate carbon as a vinyl cation now does have ~6 valence electrons (ELF indicates 5.23e). 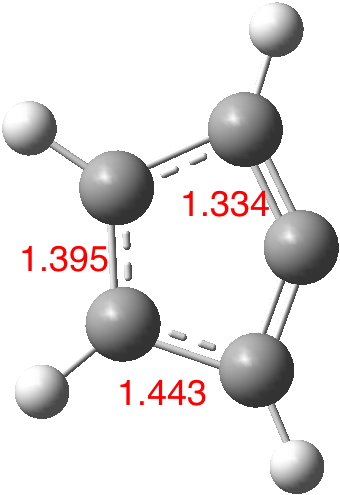
What about the other choice? By promoting two electrons from HOMO to LUMO one can also calculate 3 (again constrained to planarity)[3] which finally does correspond to the classical description of a carbene.
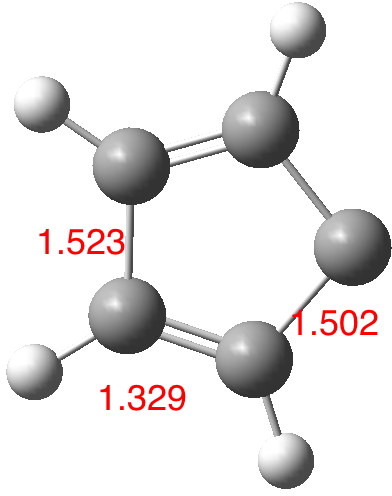
The arrow connecting 3 and 4 in the scheme at the top is NOT in this case an electronic resonance, but a a real equilibrium between two different species separated by an energy barrier. With only four π-electrons in a cycle it is also antiaromatic, and so the two localised alkene bonds avoid any conjugation with each other. This form has a free energy some 5.7 kcal/ml higher than the aromatic form. In fact, the molecule is very keen to avoid all antiaromaticity and hence if the planar constraint is lifted, it will distort with no activation to a non-planar diene (just as cyclo-octatetraene does to a non-planar tetra-ene). And to complete the tale, even though 4 is aromatic, it too distorts without activation to an odd-looking non-planar form with no symmetry[4],[5],[6] (but that is another story).
The final word should be that the naming of these types of persistent carbene does need a reality check; they should not be called this at all! They are really dipolar species or carbon-ylides as shown in 2. As it happens, a very closely related species in which one sulfur replaces one nitrogen is a very familiar compound, vitamin B1 or thiamine. The only example of a stable deprotonated thiamine derivative is referred to as a carbene[7], perhaps because with an acid catalyst it can dimerise in the manner expected of a real carbene. Significantly however, without acid catalyst this does not happen; a true carbene would not require such a catalyst.
References
- H. Rzepa, "NHC wfn", 2016. https://doi.org/10.14469/hpc/1473
- H. Rzepa, "butadiene carbene aromatic -192.700746", 2016. https://doi.org/10.14469/hpc/1581
- H. Rzepa, "butadiene carbene antiaromatic guess=alter -192.691607", 2016. https://doi.org/10.14469/hpc/1582
- H. Rzepa, "C5H4 non-planar, Cs symmetry", 2016. https://doi.org/10.14469/hpc/1583
- H. Rzepa, "C5H4 non-planar, C2 symmetry", 2016. https://doi.org/10.14469/hpc/1584
- H. Rzepa, "C5H4 non-planar, no symmetry", 2016. https://doi.org/10.14469/hpc/1585
- A.J. Arduengo, J.R. Goerlich, and W.J. Marshall, "A Stable Thiazol‐2‐ylidene and Its Dimer", Liebigs Annalen, vol. 1997, pp. 365-374, 1997. https://doi.org/10.1002/jlac.199719970213
Tags: Carbenes, chemical bonding, energy barrier, free energy, Functional groups, Ligand, Mesoionic carbene, Organometallic chemistry, Persistent carbene, quantum mechanical solution, Reactive intermediates, Transition metal carbene complex, Valence, Valence electron