Here is a little molecule that can be said to be pretty electron rich. There are lots of lone pairs present, and not a few electron-deficient σ-bonds. I thought it might be fun to look at the stereoelectronic interactions set up in this little system.
Known as ZEYDOW in the crystal structure database[1] (this species has a melting point of -138C, and its no trivial matter to measure x-ray diffraction of such a crystal!); a ωB97XD/Def2-TZVPP calculation is used to quantify the electron density [2] and this is then subjected to localisation using the ELF function. The little purple spheres represent so-called monosynaptic electron basins, or lone pairs as we might rather loosely call them (pair is not always an accurate term).
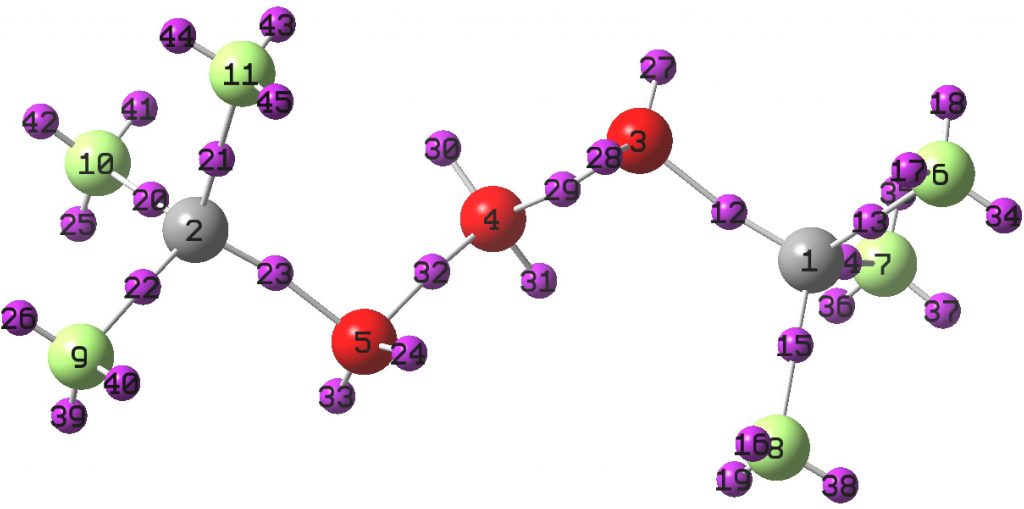
How these “lone pairs” act as electron donors into empty σ* acceptors can be quantified using NBO theory. The following table shows as many as 24 strong interactions (> 10 kcal/mol). This now augments my previous post on “Anomeric effects at carbon involving lone pairs originating from one or two nitrogens” and represents an example of “Anomeric effects at oxygen involving lone pairs originating from oxygen”.
The final two entries originate from lone pairs on the central oxygen, donating approximately antiperiplanar (~160°) into the O-CF3 antibonds, but with only a low value of the E(2) interaction energy. These two lone pairs are curiously inert.
| Lone pair donor | σ-acceptor | NBO E(2) energy |
|---|---|---|
| On F: 16,17,18,19,25,35,36,39,40,41,43,45 | C-F | 18-20 |
| On F: 26,34,37,39,42,44 | C-O | 11-18 |
| On O: 27,28,24,33 | C-F | 13-16 |
| On O: 27,33 | C-O | 13 |
| On O: 30,31 | C-O | 3.5 |
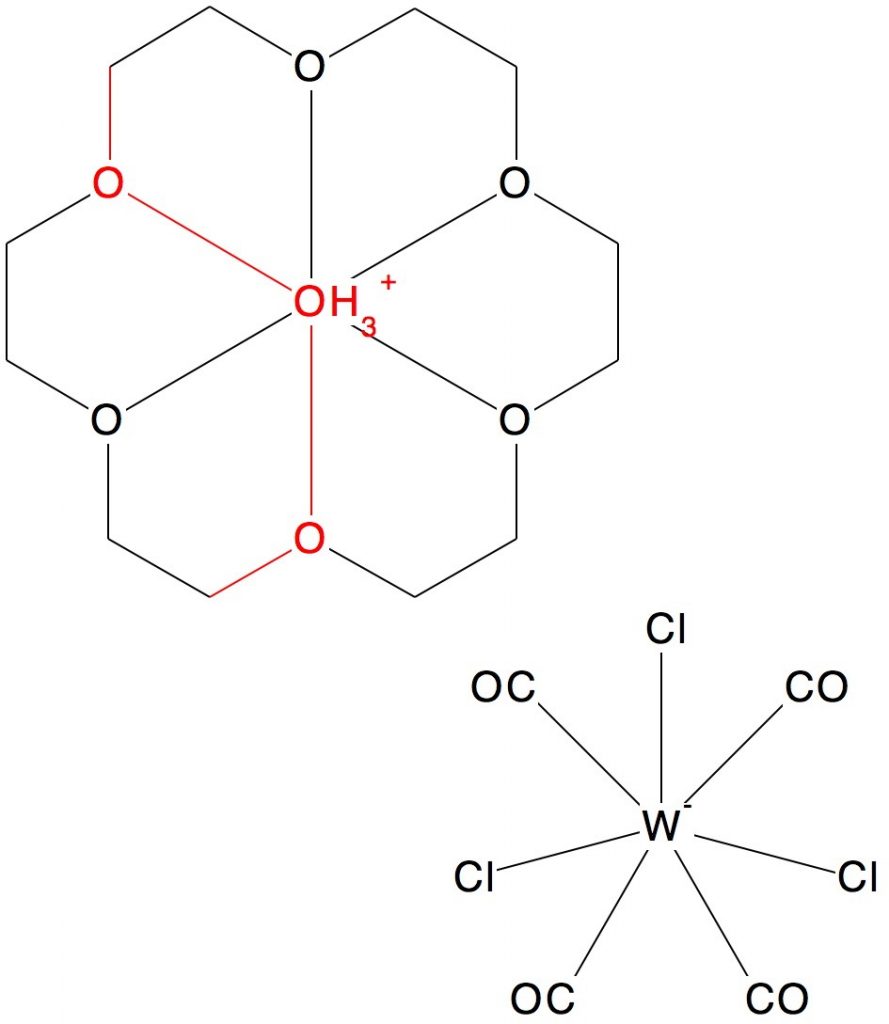
Apart from this curious molecule, there are few other examples of the R-O-O-O-R functional group,[3] but this one did catch my eye,[4] largely because it was retrieved from a search specification of R-O-O-O-R. The central oxygen apparently supports six O-O bonds, as well as three hydrogens. It is nothing of the sort of course. Reading the text reveals it is really three O…H-O bonds, disordered into two equally probable positions. There are no O-O bonds present at all, which reminds us we must always subject structures derived from x-ray crystallography to a chemical reality check.
References
- K.I. Gobbato, H. Oberhammer, M.F. Klapdor, D. Mootz, W. Poll, S.E. Ulic, and H. Willner, "Bis(trifluoromethyl)trioxide: First Structure of a Straight‐Chain Trioxide", Angewandte Chemie International Edition in English, vol. 34, pp. 2244-2245, 1995. https://doi.org/10.1002/anie.199522441
- H.S. Rzepa, "C 2 F 6 O 3", 2016. https://doi.org/10.14469/ch/195291
- Pernice, H.., Berkei, M.., Henkel, G.., Willner, H.., Arguello, G.A.., McKee, M.L.., and Webb, T.R.., "CCDC 224327: Experimental Crystal Structure Determination", 2004. https://doi.org/10.5517/cc7jfcj
- J.L. Atwood, S.G. Bott, P.C. Junk, and M.T. May, "Liquid clathrate media containing transition metal halocarbonyl anions; formation and crystal structures of [K+ · 18-crown-6][Cr(CO)5Cl], [H3O+ · 18-crown-6][W(CO)5Cl], [H3O+ · 18-crown-6][W(CO)4Cl3], and [H2O · bis-aza-18-crown-6 · (H+)2][W(CO)4Cl3]2", Journal of Organometallic Chemistry, vol. 487, pp. 7-15, 1995. https://doi.org/10.1016/0022-328x(94)05072-j
Tags: chemical bonding, Sigma bond, Stereoelectronic effect, X-ray