A while ago, I explored how the 3-coordinate halogen compound ClF3 is conventionally analyzed using VSEPR (valence shell electron pair repulsion theory). Here I (belatedly) look at other such tri-coordinate halogen compounds using known structures gleaned from the crystal structure database (CSD).
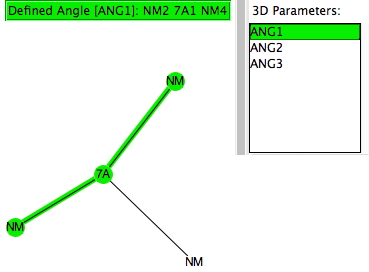
The search query specifies 7A as the central atom, defined with just three bonded (non-metallic) atoms. Initially, if no constraint on any cyclicity in the three 7A-NM bonds is made (and with R < 0.1, no errors, no disorder), the following result emerges.
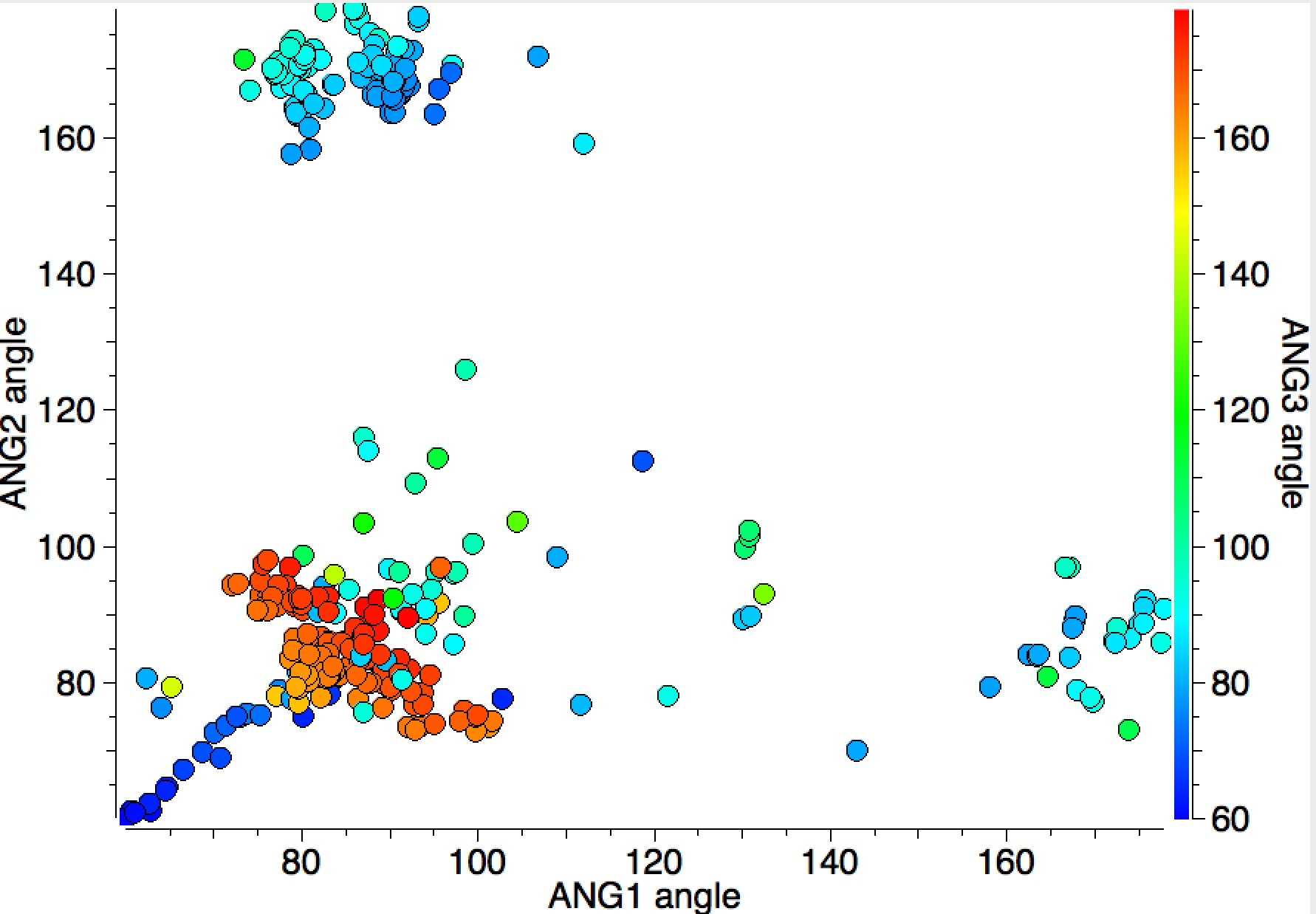
I have plotted the three angle variables using the X/Y axes above and used colour to indicate the third angle (red = ~180°, blue = ~90°). The clusters show that two of the angles are ~90° and only one is ~180°. There is also a set of blue points (~90°) which show a linear correlation and which can be shown to derive from cyclicity, as the plot below reveals when acyclicity is specified for all three NM-7A bonds.
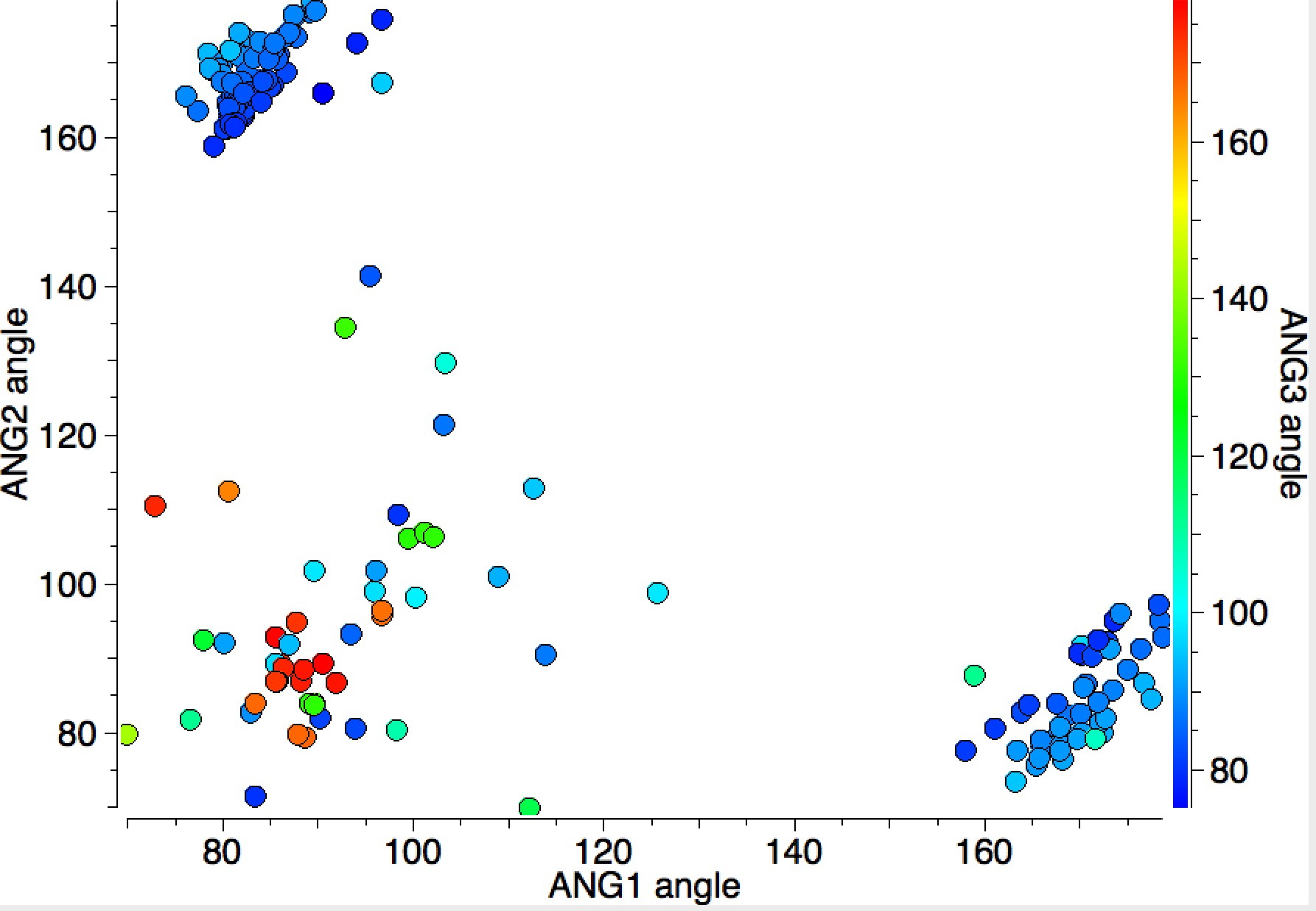
In this distribution, the two clusters for ANG1 or ANG2 of ~180° are small and compact, but the cluster where both ANG1 and ANG2 are ~90° is much more diffuse. Not all of the points in this cluster show as red (ANG3 ~180°); there are a few cyan or blue examples here too; indicating all three angles are in the range 140-90°. This result is not arising from cyclic constraints.
This wider look at 3-coordinate compounds in group 17 (the halogens) quickly reveals a class of such molecules where all three angles are relatively small. This suggests that a closer look at the bonding in these systems, especially in terms of VSEPR, might be rewarding!
I end with an equivalent search for group 18 (the noble gases). Although the number of examples is small, all show the two small/one large angle so characteristic of chlorine trifluoride itself.
The above is I think a good example of (big?) data mining, where one is searching for patterns, and if lucky spotting patterns that deviate from the norm to investigate the possibility of new chemical phenomena.[1] It is also interesting to speculate upon the origins of why two of the clusters shown above are small and compact and the third is much more diffuse.
References
- H.S. Rzepa, "Discovering More Chemical Concepts from 3D Chemical Information Searches of Crystal Structure Databases", Journal of Chemical Education, vol. 93, pp. 550-554, 2015. https://doi.org/10.1021/acs.jchemed.5b00346
Tags: chemical phenomena, data mining, equivalent search, Halogen, search query specifies 7A
The scatter diagram above when constructed for NM (non-metal) ligands surrounding the group 7A central atom shows a great deal of structure with well defined clusters at specific angles. Below I show the effect of replacing a non-metal ligand with a metal ligand (and allowing the M-7A bond to be cyclic).
It is surprisingly different with relatively few angles approaching 180°. The strongest trend is a linear correlation between the three angles, which all get larger in concert, but with two arms where it appears two angles are constant and only the third increases.