In the previous post, I explored the mechanism for nucleophilic substitution at a silicon centre proceeding via retention of configuration involving a Berry-like pseudorotation. Here I probe an alternative route involving inversion of configuration at the Si centre. Both stereochemical modes are known to occur, depending on the leaving group, solvent and other factors.[1],[2],[3]
This alternative involves attack by F– along the axial trajectory of the trigonal bipyramidal Si centre, with the OR group occupying the other axial position (TS1). In order to prepare the OR group for elimination with inversion of stereochemistry, the ion-pair complex has to reorganise (a process replacing the previous Berry pseudorotation necessary with for stereochemical retention) via TS2. And finally the OR is eliminated in TS3. The energetics of this pathway (ωB97XD/6-31+G(d) or Def2-TZVPPD/SCRF=thf) are shown below, with the inversion pathway coming out lower in energy than the previously reported retention pathway.
| System | Relative free energy | DataDOI |
|---|---|---|
| Inversion mechanism | ||
| Reactants | 0.0 | [4] |
|
TS1 |
4.9 (4.1)* | [5] |
| TS2 | 3.1 | [6] |
| TS3 | 0.0 (-0.8)* | [7] |
| Retention mechanism | ||
| TS1 | 7.9 (8.3)* | [8] |
| TS2 | 9.2 (8.7)* | [9] |
| TS3 | 5.2 (4.9)* | [10] |
* Values in parentheses are computed for the Def2-TZVPPD basis set.
The key new finding for the inversion mechanism is the ion-pair isomerisation (TS2), which is animated below. Transition states which involve no rearrangement at a bond (either formation/cleavage or rotation) are quite rare, and it is nice to show one here.
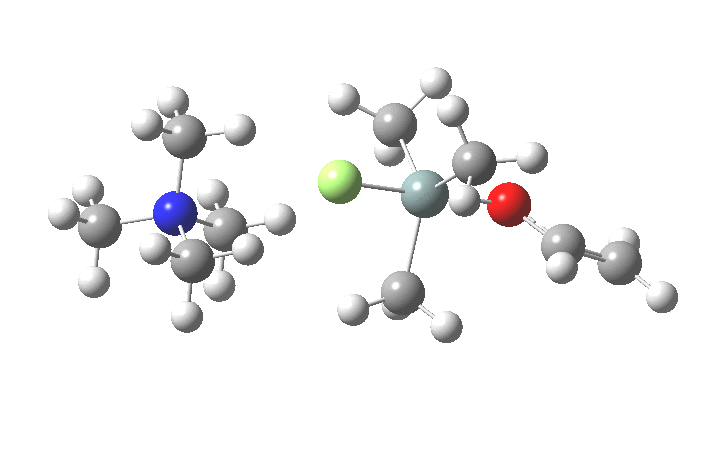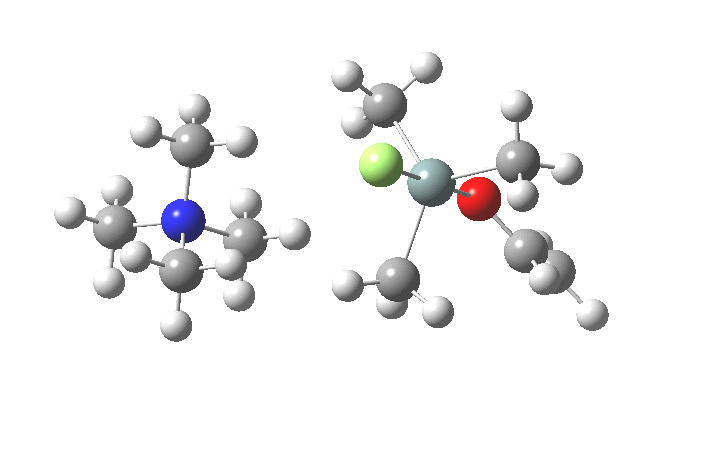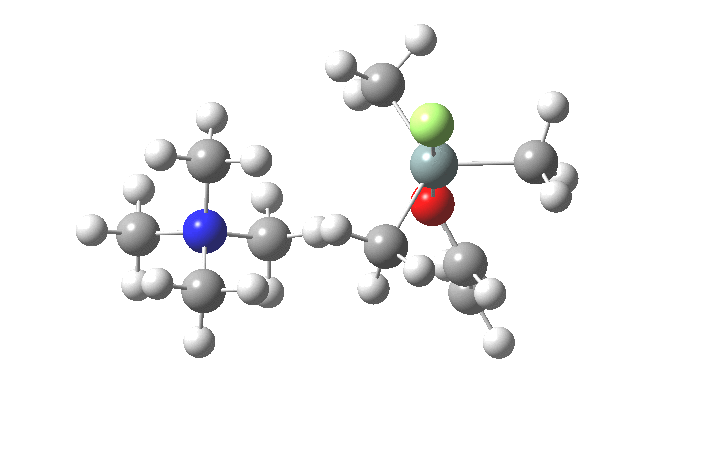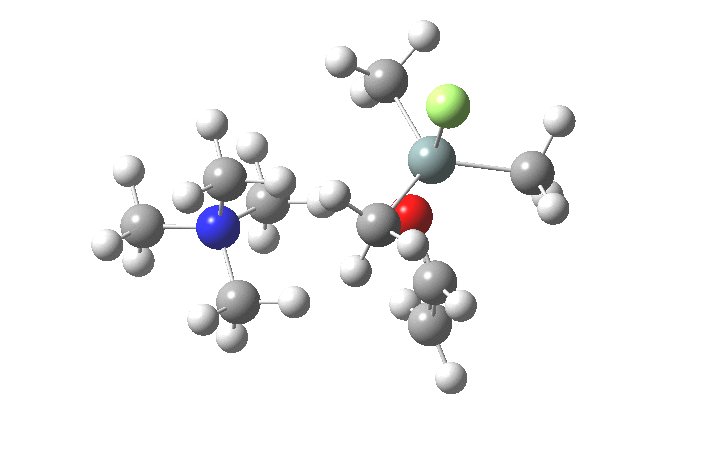
So the nucleophilic displacement reaction at 4-substituted silicon centres is really quite different from carbon.Two distinct associative/elimination mechanisms proceeding through 5-coordinate silicon seem possible. For the specific case of tetra-alkyl ammonium fluoride as nucleophile and an enolate anion as the leaving group, it appears that an inversion mechanism is favoured, and one gets strong indications of this from crystal structures of such 5-coordinate species. It might be nice to repeat this study with a reaction which is known to strongly favour retention of configuration.
References
- L. Wozniak, M. Cypryk, J. Chojnowski, and G. Lanneau, "Optically active silyl esters of phosphorus. II. Stereochemistry of reactions with nucleophiles", Tetrahedron, vol. 45, pp. 4403-4414, 1989. https://doi.org/10.1016/s0040-4020(01)89077-3
- L.H. Sommer, and H. Fujimoto, "Stereochemistry of asymmetric silicon. X. Solvent and reagent effects on stereochemistry crossover in alkoxy-alkoxy exchange reactions at silicon centers", Journal of the American Chemical Society, vol. 90, pp. 982-987, 1968. https://doi.org/10.1021/ja01006a024
- D.N. Roark, and L.H. Sommer, "Dramatic stereochemistry crossover to retention of configuration with angle-strained asymmetric silicon", Journal of the American Chemical Society, vol. 95, pp. 969-971, 1973. https://doi.org/10.1021/ja00784a081
- H. Rzepa, "enol + Me4N(+).F(-) Reactant", 2016. https://doi.org/10.14469/hpc/565
- H. Rzepa, "Di-axial elimination of F", 2016. https://doi.org/10.14469/hpc/570
- H.S. Rzepa, "C 9 H 24 F 1 N 1 O 1 Si 1", 2016. https://doi.org/10.14469/ch/195052
- H. Rzepa, "Di-axial elimination of O TS", 2016. https://doi.org/10.14469/hpc/567
- H. Rzepa, "trimethyl silyl enol + Me4N(+).F(-) 5-coordinate intermediate F axial TS", 2016. https://doi.org/10.14469/hpc/554
- H. Rzepa, "5-coordinate intermediate Berry pseudorotation TS2 New conf?", 2016. https://doi.org/10.14469/hpc/577
- H. Rzepa, "trimethyl silyl enol + Me4N(+).F(-) TS", 2016. https://doi.org/10.14469/hpc/539
Tags: Brook rearrangement, energy, free energy, Leaving group, Nucleophilic substitution, Pseudorotation, Si centre, SNi, Substitution reactions, Walden inversion