This is a corollary to the previous post‡ exploring how many molecules are needed to ionise HCl. Here I am asking how many water molecules are required to form the ionic ammonium hydroxide from ammonia and water.
As Wikipedia will inform you, “it is actually impossible to isolate samples of NH4OH (more formally the ion-pair NH4+OH– ) as these ions do not comprise a significant fraction of the total amount of ammonia except in extremely dilute solutions (my italics)”. In fact, the ionization constant Kb = [NH4+][OH–]/[NH3][H2O] is ~1.8 x 10-5 (pKb 4.75) equivalent to a free energy difference of ~6.5 kcal/mol between the two forms.† This is in stark contrast to solutions of e.g. HCl in water, where essentially all of the HCl is ionised to hydronium chloride or H3O+Cl– by addition of just ~4-5 water molecules. So what is the water model required to compute this known behaviour of ammonia? Here, this will be ωB97Xd/Def2-TZVPPD/SCRF=water, i.e. a continuum water model is already included and we add n further discrete water molecules to enhance it.
For n=0 or 2, the ion-pair is not an explicit minimum (although it appears to be a “hidden intermediate”[1]). Values of e.g. n=4,6,8 allow the formation of two or three “bridges” comprising two or three water molecules connecting the N and O atoms by hydrogen bonds and this additional solvation enables location of a transition state for proton transfer between O and N. This implies an equilibrium can be established as NH3 + H2O ⇌* NH4+.OH– with the ion-pair now a genuine minimum stabilized by those ion-pair bridges. Note in particular how the hydrogen bond lengths involving the water salt-bridge in the ion-pair are shorter than for the neutral water-ammonia complex.
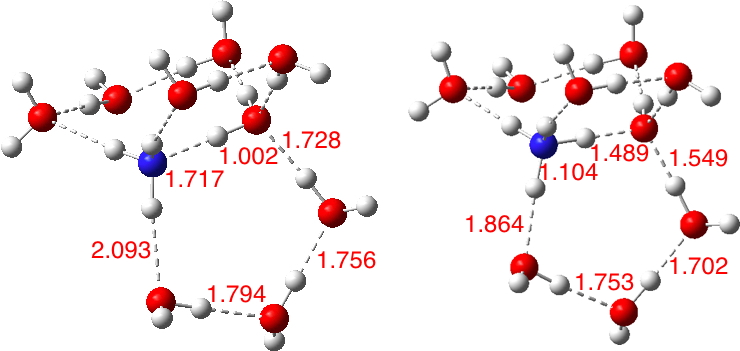
The contact ion-pair is nevertheless a very shallow minimum when surrounded by 4 or more explicit waters, the barrier from proton transfer from N being less than a vibrational quantum, and so the lifetime of the contact ion-pair is very much defined by the proton dynamics of the system..
For n=8, the dipole moment changes along the IRC for proton transfer between N and O as might be expected for the collapse of a contact ion-pair.
The relative free energies of the ion-pair and the un-ionized pair are shown below, the former being the higher. The values correspond approximately to the known ionization constant. As more explicit water molecules are added, there is a hint that the proportion of ion-pairs might actually decrease relative to neutral ammonia. However, these calculations are for a contact ion-pair and not a solvent-separated ion pair, the latter form possibly being the more appropriate form for extremely dilute solutions (see above). Modelling the latter type of ion-pair is not as straightforward as the contact variety; as the ion separation increases, so the propensity for barrierless proton transfers increases, ultimately leading back to the contact form. So to understand if it is correct that in extremely dilute solutions there is no remaining neutral ammonia, probably only a full molecular dynamics treatment of such a system is likely to give further insights.
| n | 4 | 6 | 8 |
|---|---|---|---|
| ΔΔG298 | 6.4, DOI: 10.14469/ch/191950 | 5.9, DOI: 10.14469/ch/191957 | 7.0, DOI: 10.14469/ch/191946 |
To summarise, in order to compute the formation of the ammonium hydroxide ion pair from ammonia and water, one has to include an additional four or more explicit water molecules in the calculation. This model confirms that in the resulting equilibrium, only a tiny proportion of the ammonia becomes ionised. With such a base model in place, one can now proceed to investigate how addition of substituents on the nitrogen might impact upon such ionisation, i.e. to form a stronger or a weaker base.
‡A more complete analysis followed.[2] *If you are wondering how to produce a reversible arrow, see here. †This is only approximate, since the concentration of water needs renormalising.
References
- https://doi.org/
- A. Vargas‐Caamal, J.L. Cabellos, F. Ortiz‐Chi, H.S. Rzepa, A. Restrepo, and G. Merino, "How Many Water Molecules Does it Take to Dissociate HCl?", Chemistry – A European Journal, vol. 22, pp. 2812-2818, 2016. https://doi.org/10.1002/chem.201504016
Tags: Ammonia, Ammonium, Ammonium hydroxide, Bases, dilute solutions, free energy difference, Hydroxides, Ion, Properties of water