I recently followed this bloggers trail; link1 → link2 to arrive at this delightful short commentary on atom-atom bonds in crystals[1] by Jack Dunitz. Here he discusses that age-old question (to chemists), what is a bond? Even almost 100 years after Gilbert Lewis’ famous analysis,[2] we continue to ponder this question. Indeed, quite a debate on this topic broke out in a recent post here. My eye was caught by one example in Jack's article: "The close stacking of planar anions, as occurs in salts of croconic acid …far from producing a lowering of the crystal energy, this stacking interaction in itself leads to an increase by several thousand kJ mol−1 arising from Coulombic repulsion between the doubly negatively charged anions" I thought I might explore this point a bit further in this post.
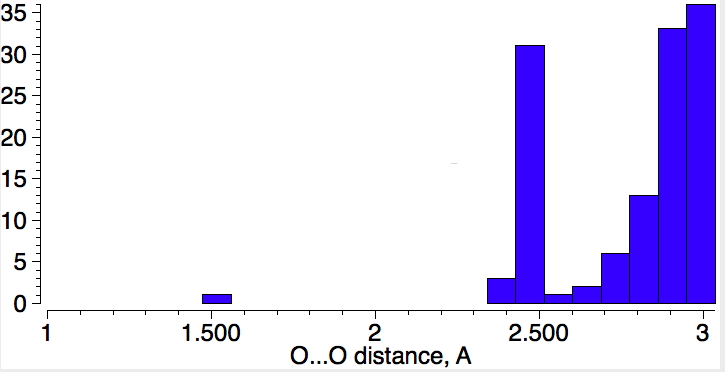
A search query of the Cambridge structure database was defined as below. Two non-bonded oxygen atoms are each attached to one carbon, each oxygen was defined as having one bonded atom (to carbon) and each assigned one negative charge. Addition of the usual constraints of R < 0.05, no errors, no disorder and specifying an intermolecular search produced 103 hits with the distance distribution shown below.
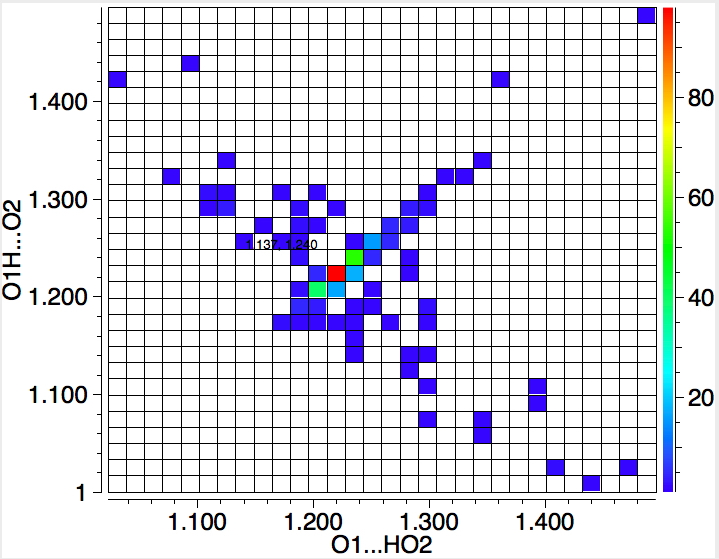
Firstly, you should be aware that the van der Waals radius for oxygen is ~1.5Å, and so any contacts less than 3.0Å become interesting. What becomes particularly exciting is the distinct cluster at ~2.5Å. Could these be ~30 examples of close encounters of the type noted by Dunitz? Well, a control search has to be done, this time for O-H-O motifs, with each OH distance plotted as below:
The hot-spot occurs when both OH distances are equal at ~1.22Å, or an O…O separation close to 2.45Å. Time to quote Dunitz again "This large destabilization is, of course, more than compensated in the overall energy balance by the large stabilization arising from Coulombic interactions of the croconate anions with the surrounding cations." In this case of course, the cation is a proton, residing at the half way point between the two oxygens. So two oxygens can indeed approach ~0.5Å closer than the sum of the vdw radii if a proton sits in-between them.
What do we learn? Well, firstly that one should always have a reality check of the results of any crystal structure search. The search did specify that the oxygens be non-bonded but also that they should both carry a negative charge and that both should only have one bonded atom. That should in theory at least have excluded any C-O-H-O-C structures, so why were about 30 such examples found? I can only speculate here, but recollect that 50 years ago when the CSD was founded, hydrogen atoms were rarely identified from the electron density. They were instead placed or "idealised" to where they might be expected. Nowadays any contentious hydrogens are almost always located rather than idealised, but clearly their status as bona-fide atoms is not quite so strong as the rest of the periodic table. So in at least some of these 30 examples with short O…O contacts, we might expect there to lurk a (possibly unrecognised) proton. But one never knows, there may be some real examples of O…O contacts with no such proton intervening. Now these really would be interesting.
Postscript. F is isoelectronic with O(-); below is the same search as defined above, but for non-bonded CF…FC approaches. 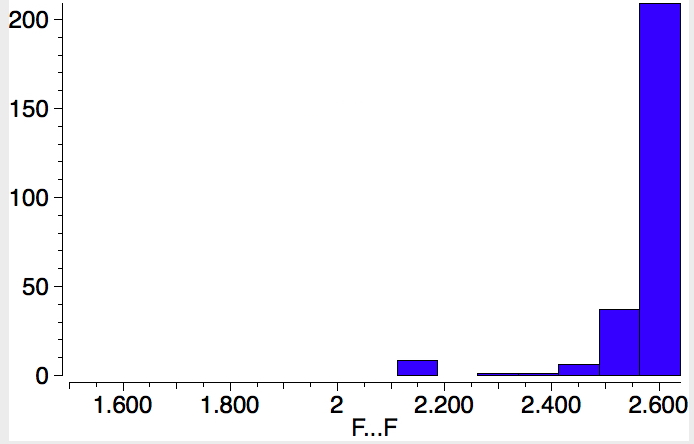
The vdw radius of F is 1.45Å hence any non-bonded contact <2.9Å is worth taking a look at. But notice the small cluster of about 10 compounds for which the value is ~2.15Å. The F-H-F plot shows a hot spot at ~2.3 for the F…F separation, but there are zero hits for CF-H-FC. So these ten hits are indeed tantalising.
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
References
- J.D. Dunitz, "Intermolecular atom–atom bonds in crystals?", IUCrJ, vol. 2, pp. 157-158, 2015. https://doi.org/10.1107/s2052252515002006
- G.N. Lewis, "THE ATOM AND THE MOLECULE.", Journal of the American Chemical Society, vol. 38, pp. 762-785, 1916. https://doi.org/10.1021/ja02261a002
Tags: Carbon, Cations, Chemical bond, control search, Croconic acid, crystal energy, crystal structure search, Gilbert Lewis, intermolecular search, Jack Dunitz, overall energy balance, Proton

Samantha Jenkins found evidence of O…O interactions in high-pressure phases (Ice-VI, Ice-VII, Ice-VIII) of H2O ice in early papers. In particular, between O atoms in different sublattices. See, for example, the bibliography at http://www.beaconresearch.org/#%5B%5BProf.%20Samantha%20Jenkins%5D%5D, specifically:
“The Importance of O–O Bonding Interactions in Various Phases of Ice”, In Physics and Chemistry of Ice, 257–264. Bremerhaven, Germany: Royal Society of Chemistry, 2006. DOI: 10.1039/9781847557773
Earlier work:
Journal of Physics: Condensed Matter 13(41) 9207-9229 (2001) DOI: 10.1088/0953-8984/13/41/312 .
Journal of Physical Chemistry B 103(50) 11041-11049 (1999) DOI: 10.1021/jp992655w .
Chemical Physics Letters 317(1-2) 97–102 (2000) DOI: 10.1016/S0009-2614(99)01306-8.