Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[1]. 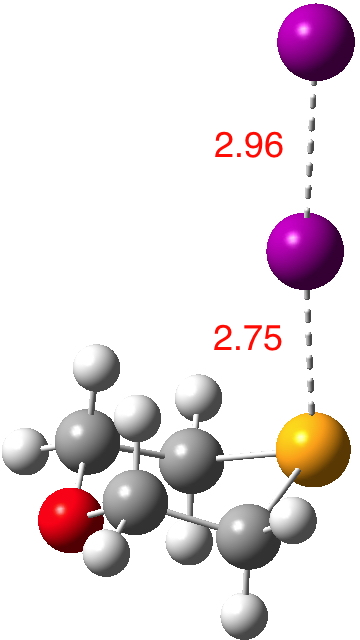 The features of interest include:
The features of interest include:
- The six-membered ring is in the chair conformation.
- The (relatively enormous) I…I substituent is axial!
- It is attached to the Se rather than the O.
- The Se…I distance is 2.75Å, some 1.13Å shorter than the combined atom ver der Waals radii (1.90 + 1.98 = 3.88)
- The Wiberg bond index is 0.42 for the Se-I bond and 0.63 for the I-I bond (at the crystal geometry). It is tending towards a symmetrical disposition of the central iodine (a feat also achieved by the iodine in the NI3 complex).
- The NBO E(2) perturbation involving donation from the Se lone pair into the I-I antibond is 77 kcal/mol, almost twice the value of the one involving DABCO…I-I and way above the values found for the related hydrogen bond.
- The B3LYP+D3/Def2-TZVPP+PP(I) optimised structure expands the Se-I bond distance to 3.04 and contracts the I-I distance to 2.81Å indicating (as with DABCO…I-I) that there may be compression of this bond induced by the lattice.
-
The NCI surface shows a classical "strong" interaction between Se and I (blue), but significant additional features arising from the axial hydrogens that might account for the axial orientation of the Se…I-I group.
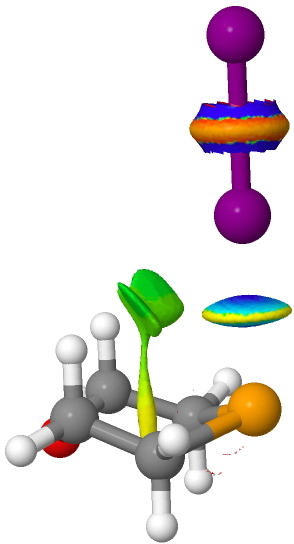
Click for 3D
-
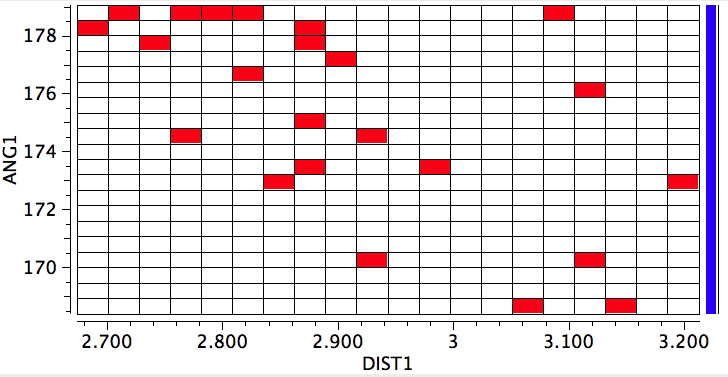
For good measure, here is the complete crystal structure search, defining any non-bonded contact between any element of group six and group seven that is <0.5Å shorter than the van del Waals contact. Our candidate is on the left hand edge of the plot.
References
- H. Maddox, and J.D. McCullough, "The Crystal and Molecular Structure of the Iodine Complex of 1-Oxa-4-selenacyclohexane, C<sub>4</sub>H<sub>8</sub>OSe.I<sub>2</sub>", Inorganic Chemistry, vol. 5, pp. 522-526, 1966. https://doi.org/10.1021/ic50038a006
Tags: chair, crystal structure search
Per-Ola Norrby @PeONor tweeted “Why is not I3– the strongest halogen bond? Electronically identical, and a charge shouldn’t matter”
He certainly has a point! Although in fact the charge might matter. For a charged nucleophile, the charge gets delocalised as a result of halogen bonding. But for a neutral nucleophile, charge gets separated instead. Charge separation tends to require stabilisation (solvent, crystal lattice, dispersion).
Thus I noted above that a B3LYP+D3/Def2-TZVPP calculation on the isolated molecule resulted in a Se…I bond length of ~3.04Å. Repeat the same calculation in a solvent field (water), in this case acting as a proxy for a crystal lattice, and the predicted length goes down to 2.83Å (and the I-I distance increases to 2.94Å, DOI: 10.6084/m9.figshare.1263627).
So perhaps one should instead change the above to ask if the Se…I system is the strongest neutral halogen bond?
Lars Öhrström @Larsohrstrom tweeted “Or there are bond length isomers, I3– and I2…I- ??”
Here is a plot of the two distances in I-I-I for a number of different structures. Although the red hotspot is symmetric, the distribution around it is not circular but “X-shaped”, which suggests that asymmetric variations are also possible. The top left is strongly asymmetric, with an I…I distance quite similar to Se…I.
Here is another plot, this time using “any halogen” for the search rather than specifying I. Both I and Br seem to exhibit the same feature.
A quick follow up to my noting that the distribution of I-I-I bond lengths was “X-shaped”.
The left leaning arm of this X charts the asymmetry in the two lengths. The less prominent right leaning arm charts the equal “compression/elongation” of both bonds. These two motifs are a bit like the asymmetric and symmetric stretches in eg O=C=O.
I fancy this “X-shaped” response might be quite rare! I wonder where else one should look for it?
To answer the question posed at the end of the last comment, here is a search of X-H-X (X =any group six or seven element).
The left leaning arm of the X is now very prominent (search for R factor < 0.05, no errors, no disorder and normalised H-positions). There is a hint of a right leaning arm, but not so as you would have total confidence.
Because the left leaning arm is evenly distributed, we may assume that it probably does not represent a double minimum, but merely one asymmetric minimum. But more analysis would be needed to confirm that.
Dear Prof. Rzepa,
we found almost equal calculated Se-I and I-I bond length for our in-silico compound no. 11 here:
10.1002/chem.201100970 (see Support info)
The Wiberg index (optimized structure) were 0.31 and 0.21, respectively.
best regards
Henry,
when its about [I3]- isn’t it enough to say that its a completely orbital based covalent interaction? And doesn’t that exclude what is commonly understood as “halogen bond”?
Raphael,
I thought of it rather differently. Whilst I3– could be regarded as a covalent interaction, fundamentally I do not think it is any different from a strong hydrogen bond, O-H-O where the proton is symmetrically disposed between the two oxygens. We still call this a hydrogen bond, even though it could also be described as a covalent three-centre-two-electron bond. There are strong symmetrical H-bonds, and weaker asymmetrical ones covered by the same name. With the halogens, I am simply applying the same principle.