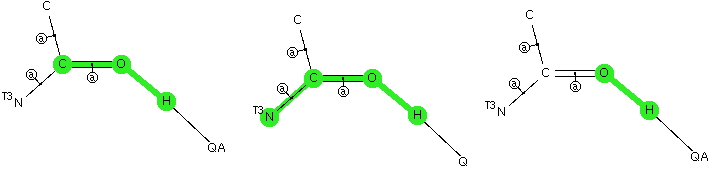
The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack. My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder).
 The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl)
The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl) 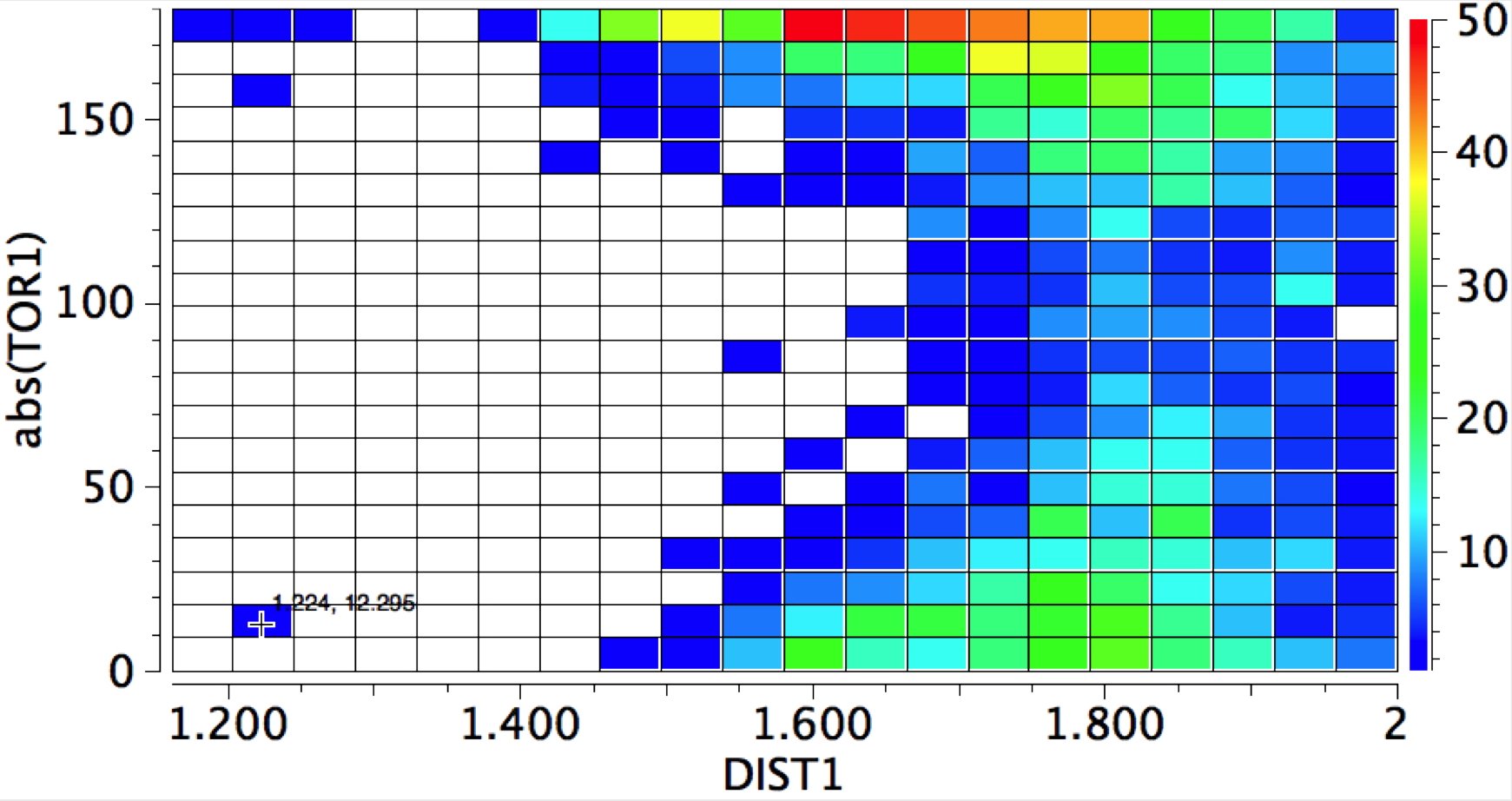
- The first observation is to note the prominent "hotspot" at a torsion of 180° and a (hydrogen bonding) distance of ~1.60-1.65Å. Amides, so it seems, prefer the electrophile (a proton) to approach anti to the nitrogen
- There is a smaller hotspot at a torsion of 0° and a rather longer distance of ~1.8Å corresponding to syn approach.
- And finally a barely discernible (but real) one at ~90°, corresponding to the proton attaching itself to the carbonyl π-bond.
-
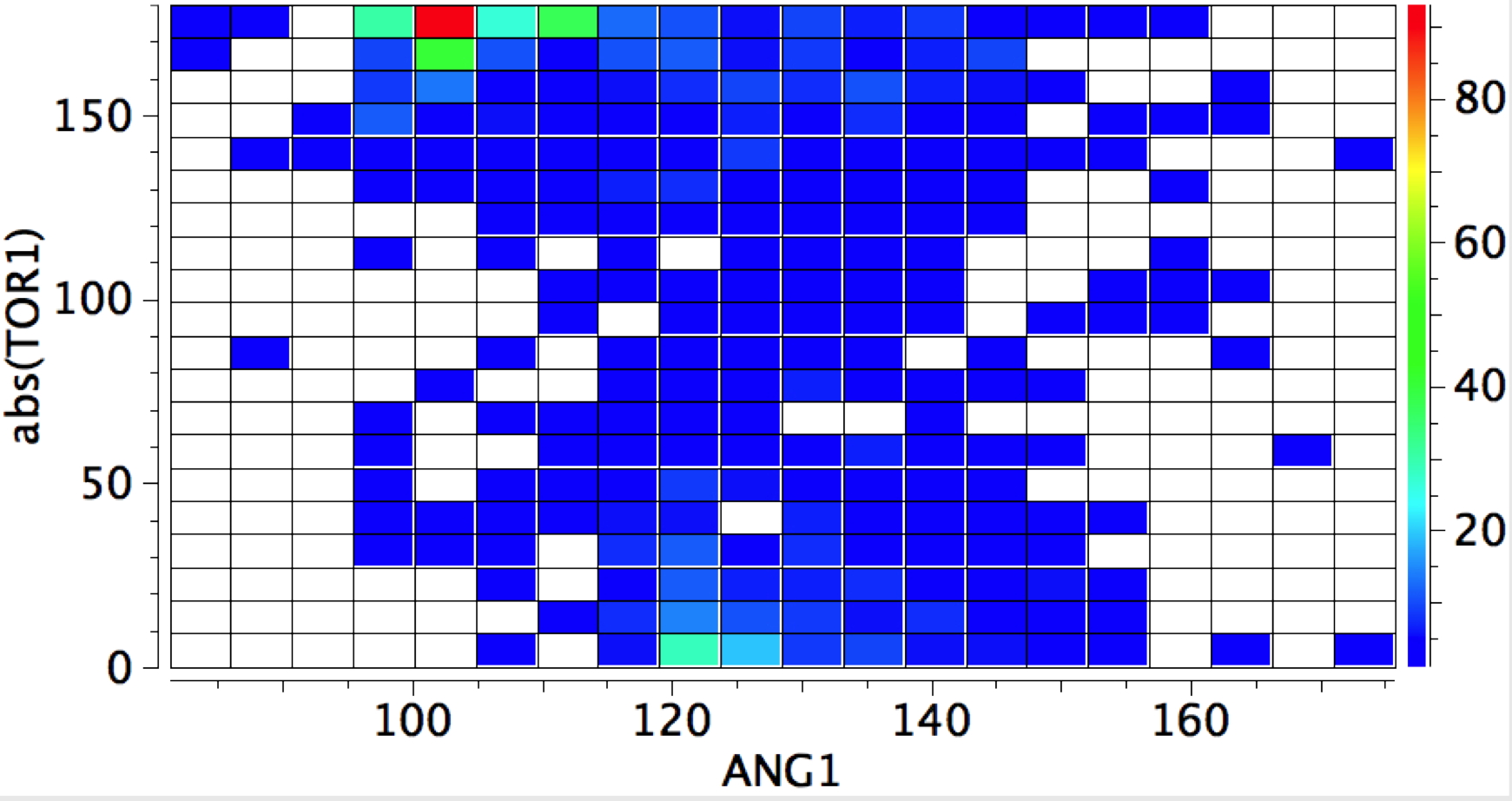
A plot of the angles involved reveals that the anti hotspot occurs at ~100° whilst the syn hotspot is about 120°.
-
whilst replacing the proton as electrophile by any metal results in a distinct change.
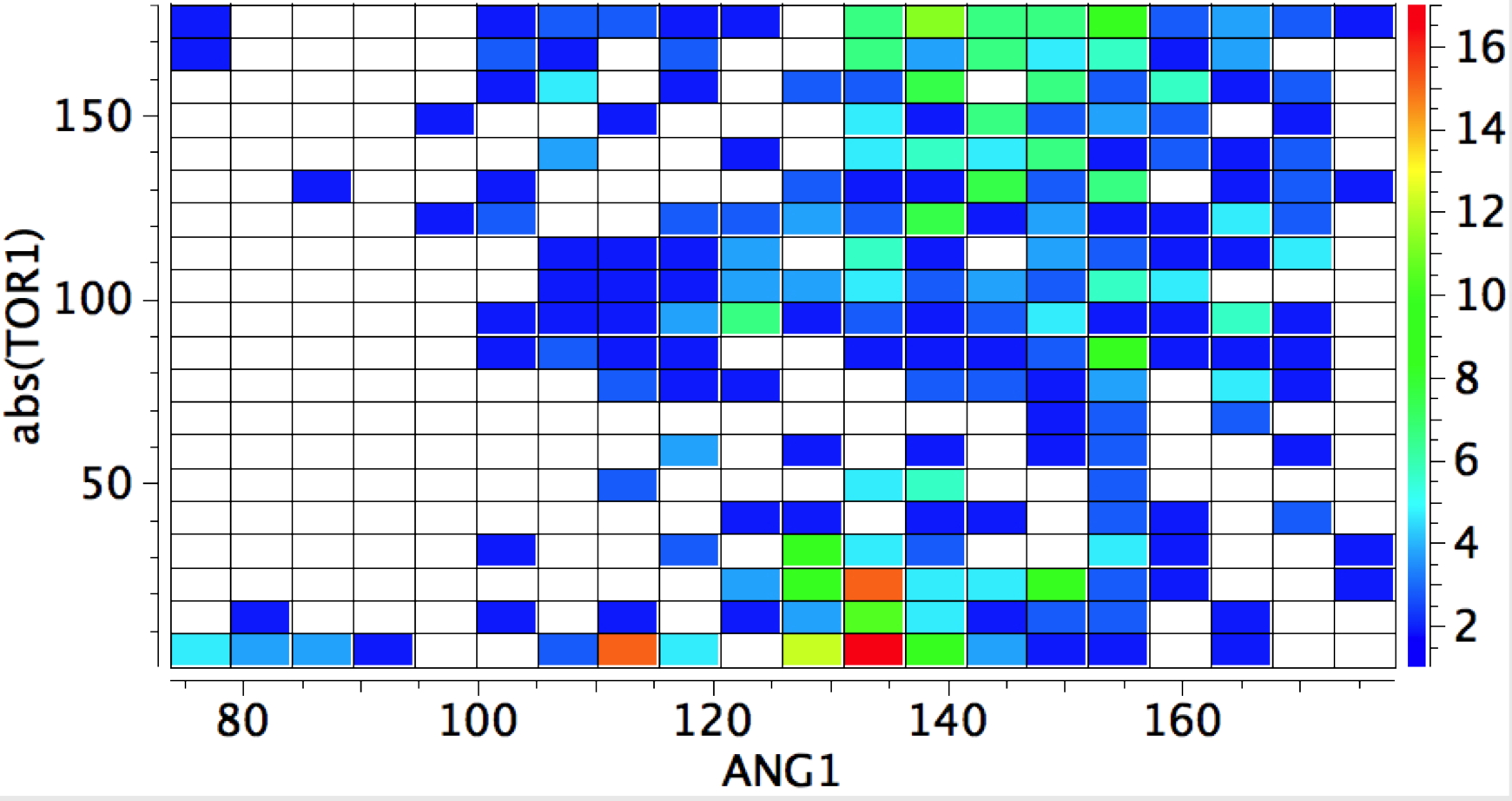
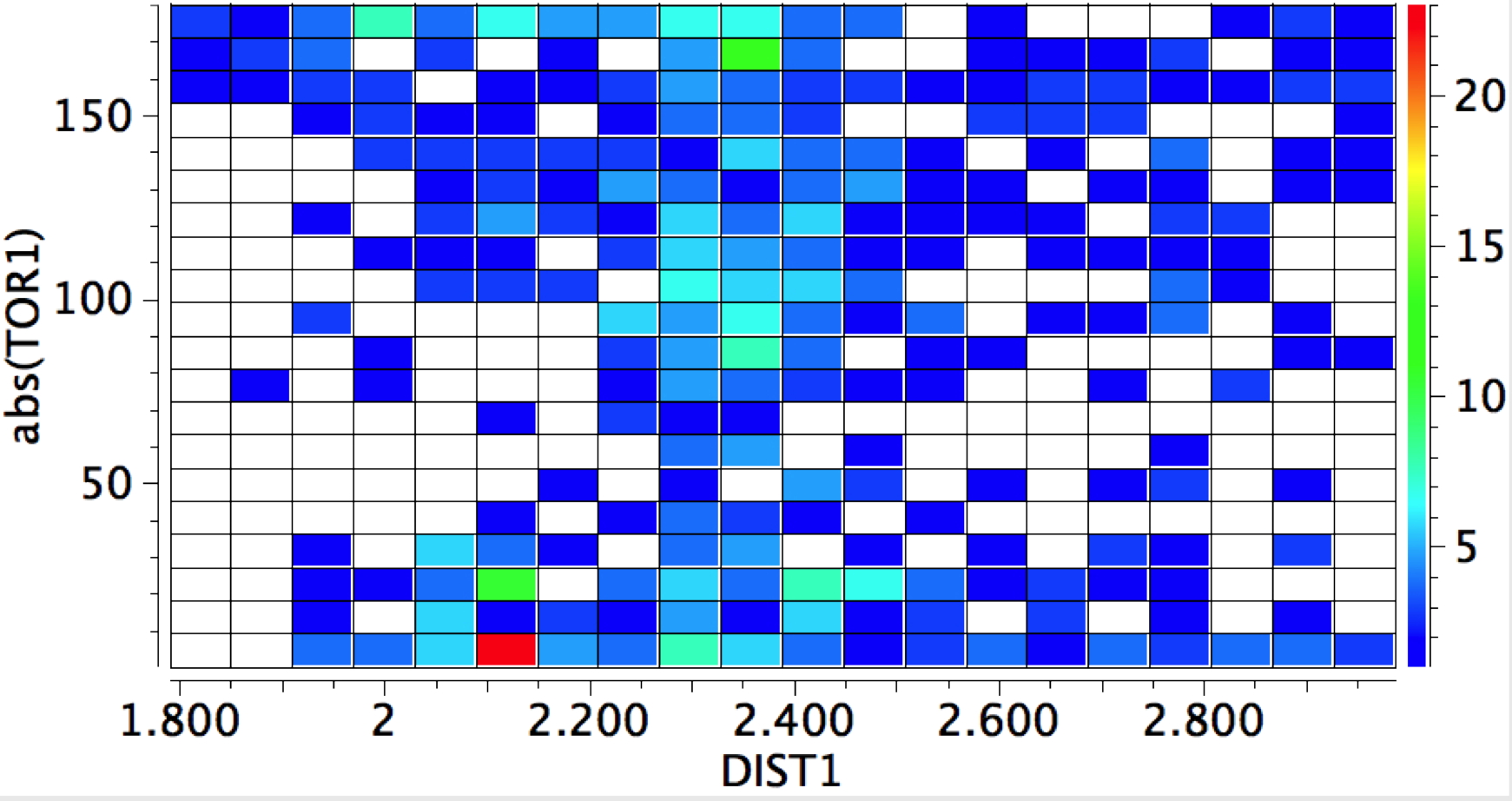
- Syn approach now holds the (red) hotspot, and the angle opens up to ~135°, whilst the anti approach covers a wider angle range of 130-150°
- A third hotspot region occurs for the 90° torsion, again metal-π-bond interactions.
The above is a very general statistical survey. As with most bonding effects, one really should investigate every example to discover any perturbing circumstances or structural motifs that might distort the outcome. But for a ten minute exercise in response to a fascinating question from a colleague, it's not bad! And it certainly nicely inverts the usual Bürgi–Dunitz view of carbonyl groups.
Tags: electronics, energy, metal results, metal-π-bond interactions, search definition