Homoaromaticity is a special case of aromaticity in which π-conjugation is interrupted by a single sp3 hybridized carbon atom (it is sometimes referred to as a suspended π-bond with no underlying σ-foundation). But consider the carbene shown below. This example comes from a recently published article[1] which was highlighted on Steve Bachrach’s blog. Here aromaticity has resulted from a slightly different phenomenon, whereby a 4π-electron planar (and hence nominally anti-aromatic) molecule is elevated to aromatic peerage by promoting the two carbene σ-electrons to have π-status.
Normally, such electrons in a σ framework are considered to be more stable than in a p-π-framework,‡ because the s-character of the former does not have a node at the nucleus and hence the electrons are bound more strongly. In this case however, the transformation from anti-aromaticity to aromaticity provides more than enough stabilisation through resonance for the σ-to-π promotion to occur.
So here I ask a different question to that posed on the aforementioned blog or article; could you achieve the same result using ten electrons rather than six? On the left of the diagram above for the 10-case, we have a planar 8-ring with 8 p-π-electrons and two carbene electrons. Promoting the latter would produce a 10π-aromatic ring (for another example of such, see here). Indeed so![2] This 10π-system has five occupied π-MOs, a low energy delocalised σ-MO of interest (below; is it σ-aromatic?) and a LUMO corresponding to the vacated carbene orbital.
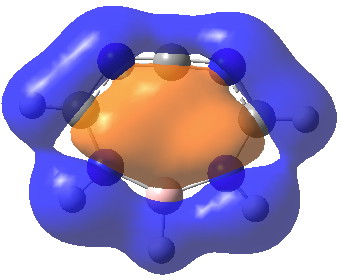
Click for 3D
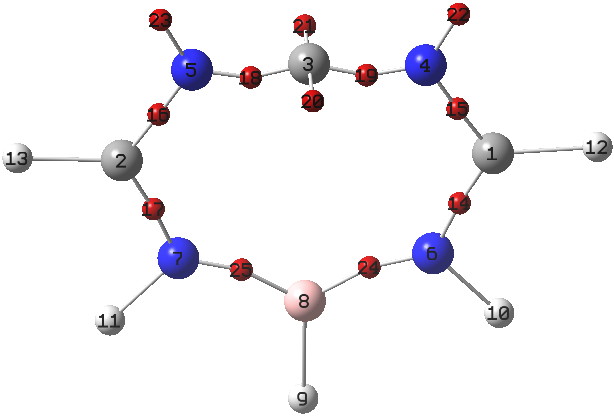
ELF analysis (below) also reveals no carbene lone pair (basins 20 and 21 have 0.44e each, and 18/19 2.70 each, giving that central carbon 6.28e, resembling the vinyl carbocation shown above in resonance).
Click for 3D
Not all is rosy however. This species is in fact not a stable minimum, but a transition state interconverting two buckled (and hence non-aromatic) conformations. It seems that the additional angular ring strain induced by an 8-membered ring has pushed it over the edge from aromatic royalty to non-aromatic commoness (remember this?). But that apart, we can see that 10 electrons can behave similarly to six in inducing a two-electron promotion from a carbene lone pair. Not quite homoaromaticity; I think it should be given its own named aromatic type! Any suggestions?
‡That is certainly largely true of carbon. But much less true for the more electropositive boron. Thus it is not unusual to find such promotions occurring for planar boron frameworks.
References
- B. Chen, A.Y. Rogachev, D.A. Hrovat, R. Hoffmann, and W.T. Borden, "How to Make the σ<sup>0</sup>π<sup>2</sup> Singlet the Ground State of Carbenes", Journal of the American Chemical Society, vol. 135, pp. 13954-13964, 2013. https://doi.org/10.1021/ja407116e
Tags: low energy, Steve Bachrach
[…] Chemistry with a twist « Six vs ten aromatic electrons? […]