The title of this post comes from a comment posted by Ryan, who asks about isocyanide’s role (in the form of the anion of tosyl isocyanide, or TosMIC) in two named reactions, Van Leusen and Ugi FCR. “In Van Leusen, it (the isocyanide) acts as an electrophile: however, in Ugi, it acts as a nucleophile”. Here are some valence bond forms for this species;
Thus we see representation 3 as a vinyl carbene, and because the carbon is isoelectronic with a carbocation, it could be regarded as an electrophile (with donation into an empty p-orbital). With 2, we see that that this anion is actually isoelectronic with diazomethane 4, a species that can act as a 1,3-dipole in cycloaddition reactions. Form 2 is also of interest in another context; the terminal carbon can be regarded as using none of its four valence electrons for bonding (having them all in two lone pairs). Frenking has described such species as divalent C(0), or carbones[1]. With 1, we see isoelectronic analogy to the acetylide anion, where the terminal carbon is nucleophilic. So three different personalities, and as is often the case, such chameleon character can also reflect in the reactions.
Because 1-3 effectively differ in how the two lone pairs are distributed, it is useful to analyse the actual character using a method which can locate and count them. So here is an ELF analysis[2] which can provide the centroids of electron basins together with the required integration.
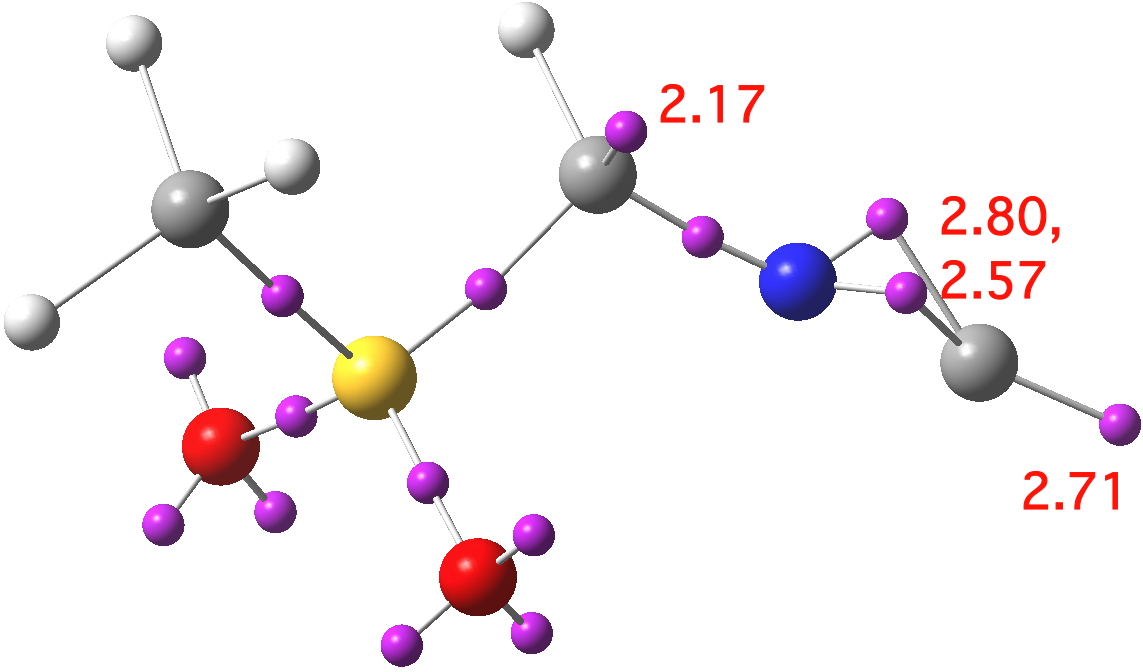
Click for 3D
This reveals 1 as the closest match. Thus the terminal carbon has two disynaptic basins and one monosynaptic basin corresponding to 2.71 electrons giving a total valence electron count of 8.08 (which excludes 3), rather closer to acetylide 1 than to the carbone 2. Another lone pair is located on the first carbon (2.17) which again matches structure 1, and that carbon is indeed strongly pyramidal, and presumably also strongly nucleophilic. A similar conclusion emerges from NBO analysis, which reveals two π-type NBOs and one terminal lone pair NBO on the isocyanide group, indicating the acetylide form.
I will deal with whether the reactions of this species reflect the nucleophilic or electrophilic character of the isocyanide group in a separate post.
References
- H.S. Rzepa, "Gaussian Job Archive for C4H6NO3S(1-)", 2013. https://doi.org/10.6084/m9.figshare.706756
Tags: Ugi, Van Leusen
[…] Chemistry with a twist « How should one represent the anion of TosMIC? […]
Hi Henry, Thanks so much for your kindly analysis.It’s great to know the structure 1 is the closest one to the reality. When I look at the 3D modol,I’m a bit confused by the electrons around the terminal carbon. It has only three pair of electrons, however, the total valence count is 8.