Lukas, who occasionally comments on this blog, sent me the following challenge. In a recent article[1] he had proposed that the stereochemical outcome (Z) of reaction between a butenal and thioacetic acid as shown below arose by an unusual concerted cycloaddtion involving an S-H bond. He wrote in the article “…this scheme … recommends itself for evaluation by in silico methods“. I asked if the answer could be posted here, and he agreed. So here it is.
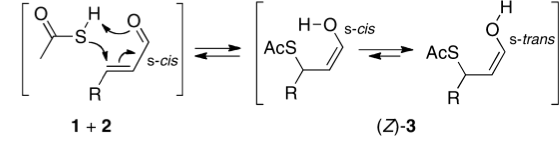
Click for 3D
My initial instinct was that it might prove to be a reaction with “hidden intermediates” (for example transfer of the S-H proton onto the oxygen to form a zwitterionic “hidden intermediate”). Well, this is what the computed transition state[2] and the IRC[3] look like.
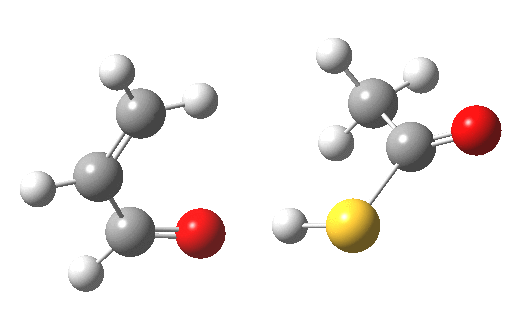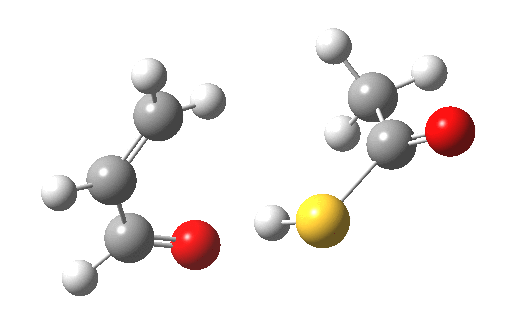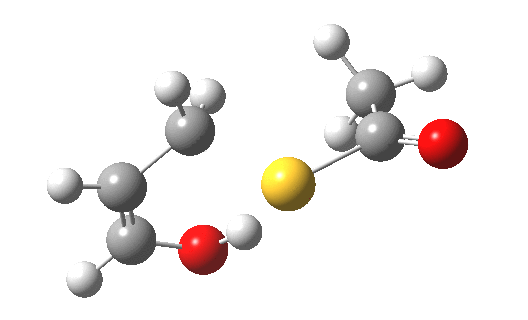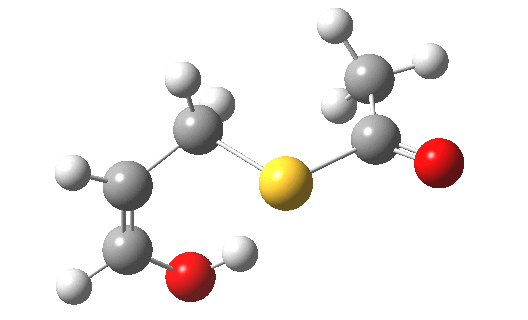
- For acrolein itself, the forward free-energy barrier ΔG†298 is 25.8 kcal/mol (ωB97XD/6-311G(d,p)/SCRF=acetone) and the reverse barrier 27.7 kcal/mol, which makes the overall free energy for the reaction -1.8 kcal/mol. These values are not inconsistent with an equilibrium thermal reaction at room temperatures or above.
- The gradients show no sign of any “hidden intermediates”. The reaction indeed is nicely concerted, albeit with O-H bond formation asynchronously preceding that of C-S. This reaction should indeed be added to the pantheon of facile pericyclic (or pseudopericyclic) reaction types.
- For the methyl substituted system (R=Me) the forward barrier is a little lower than before (23.8 kcal/mol)[4] and the reverse likewise (25.5).‡ The (Z) product far dominates the (E) (61:1). The IRC[5] is similar to the unsubstituted reaction, but with the faintest of hints of a hidden intermediate (at IRC ~0.8).
The reaction itself is a smaller-ring thia-homologue of one reported by Birney[6] and classified there as a pseudopericyclic reaction, the point of interest being the difference in behaviour between the O-H acid and the S-H acid.
Well, that is the in silico counterpart to the in silica experiment. It took a few hours (about the same as a few NMR measurements?).
‡Slightly lower values are obtained for the s-cis conformation[7] of the thio-acid: ΔG†298 is 21.0 kcal/mol for the forward and 22.0 for the reverse reactions.
References
- L. Hintermann, and A. Turočkin, "Reversible Generation of Metastable Enols in the 1,4-Addition of Thioacetic Acid to α,β-Unsaturated Carbonyl Compounds", The Journal of Organic Chemistry, vol. 77, pp. 11345-11348, 2012. https://doi.org/10.1021/jo3021709
- H.S. Rzepa, "Gaussian Job Archive for C5H8O2S", 2013. https://doi.org/10.6084/m9.figshare.701466
- H.S. Rzepa, "Gaussian Job Archive for C5H8O2S", 2013. https://doi.org/10.6084/m9.figshare.701174
- H.S. Rzepa, "Gaussian Job Archive for C6H10O2S", 2013. https://doi.org/10.6084/m9.figshare.701467
- H.S. Rzepa, "Gaussian Job Archive for C6H10O2S", 2013. https://doi.org/10.6084/m9.figshare.701468
- H. Ji, L. Li, X. Xu, S. Ham, L.A. Hammad, and D.M. Birney*, "Multiphoton Infrared Initiated Thermal Reactions of Esters: Pseudopericyclic Eight-Centered<i>cis</i>-Elimination", Journal of the American Chemical Society, vol. 131, pp. 528-537, 2008. https://doi.org/10.1021/ja804812c
- H.S. Rzepa, "Gaussian Job Archive for C6H10O2S", 2013. https://doi.org/10.6084/m9.figshare.701501
Tags: Reaction Mechanism
Henry, thanks a lot, this is very nice. The statement “…this scheme … recommends itself for evaluation by in silico methods“ had been partly inspired by your blog.
It is good to know that the concerted mechanism is “making sense” here. In view of the stereochemical result (for crotonaldehyde), this makes me believe that the concerted mechanism is truly operating.
In case of acrolein, there must be another open-chain mechanism operating in parallel, which leads to some (E)-product in acetone (E/Z ≈ 1:4) or considerably more in polar donor-solvents (MeOH, E/Z = 2:1; DMSO, 6:1). The lower barrier for the concerted mechanism with crotonaldehyde is an interesting result, which I would not have expected, by intuition.
Perhaps kinetic isotope effects (2H, 13C ?) might further probe whether the reaction for either system is concerted or open-chain?
I have computed kinetic isotope effects for 2H replacing S-H and for 13C on the carbon where a S-C bond forms for the crotonaldehyde reaction (R=Me). I would first note that
1. The S-H-O angle is 162°, it is almost linear, despite being part of a 6-membered ring.
2. At the transition state, the proton has essentially completed transfer (rSH 2.007Å, rOH 1.023Å), and it is the C-S bond that is actually forming.
The KIE come out as follows:
1H/2H = 1.76 (small)
12C/13C 1.024 (medium).
If this can be verified by experiment, it would strengthen the case for the concerted 1,4 addition across the S-H bond.