In the previous post, I explored the so-called “impossible” molecule methanetriol. It is regarded as such because the equilbrium resulting in loss of water is very facile, being exoenergic by ~14 kcal/mol in free energy. Here I explore whether changing the substituent R could result in suppressing the loss of water and stabilising the triol.
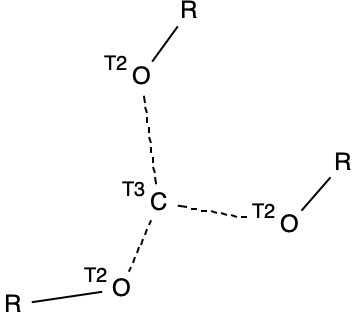
I started (as I usually do) with a search for crystal structures, in this case containing the motif shown below (trisubstituted carbon, disubstituted oxygen and R = H or C and any type of connecting bond), which is the species resulting from loss of R– to form a trihydroxycarbenium cation.