In the previous post, I explored the so-called “impossible” molecule methanetriol. It is regarded as such because the equilbrium resulting in loss of water is very facile, being exoenergic by ~14 kcal/mol in free energy. Here I explore whether changing the substituent R could result in suppressing the loss of water and stabilising the triol.
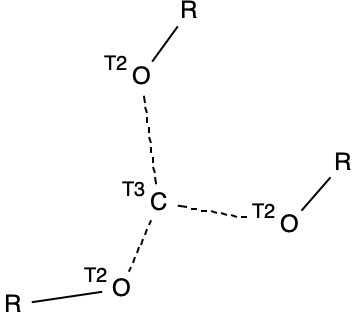
I started (as I usually do) with a search for crystal structures, in this case containing the motif shown below (trisubstituted carbon, disubstituted oxygen and R = H or C and any type of connecting bond), which is the species resulting from loss of R– to form a trihydroxycarbenium cation.
Archive for May, 2024
Possible Formation of an Impossible Molecule?
Monday, May 20th, 2024Exploring Methanetriol – “the Formation of an Impossible Molecule”
Thursday, May 16th, 2024What constitutes an “impossible molecule”? Well, here are two, the first being the topic of a recent article[1]. The second is a favourite of organic chemistry tutors, to see if their students recognise it as an unusual (= impossible) form of a much better known molecule.
References
- J.H. Marks, X. Bai, A.A. Nikolayev, Q. Gong, C. Zhu, N.F. Kleimeier, A.M. Turner, S.K. Singh, J. Wang, J. Yang, Y. Pan, T. Yang, A.M. Mebel, and R.I. Kaiser, "Methanetriol─Formation of an Impossible Molecule", Journal of the American Chemical Society, vol. 146, pp. 12174-12184, 2024. https://doi.org/10.1021/jacs.4c02637