Stereoelectronics: Tutorial Problems
Question 2
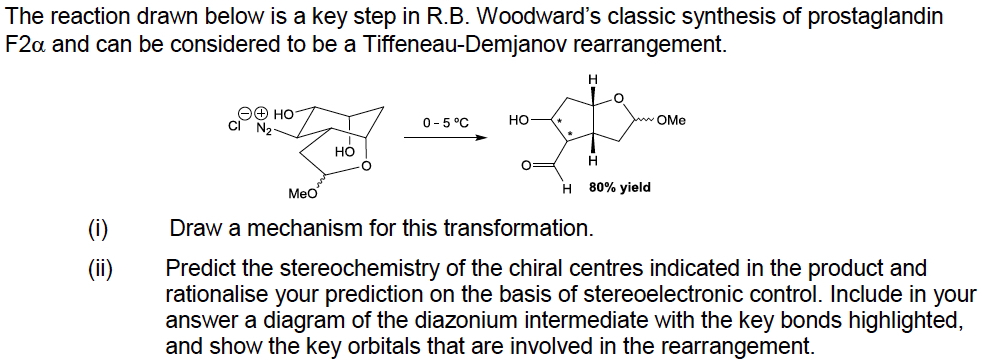
Answer: The diazonium ion is evicted from the system by one of two different sets of anti-periplanar interactions involving a C-C bond. The higher energy route (orbital overlap; purple overlaps with blue, orange overlaps with red, click Show to reveal) migrates this as promoting a process which populates the C-N σ* region to induce its departure.
The lower energy route (orbital overlap; purple overlaps with blue, orange overlaps with red, click Show to reveal) migrates this with a better antiperiplanar alignment, producing a carbonium/oxenium-like hidden intermediate after eviction of N2 which looks like a π-complex. It can also be considered similar to the 3-centre-two electron non-classical carbonium ion found for the norbornyl cation. This π complex/non-classical ion inhibits free rotations and hence is the mechanism that ensures stereochemical integrity. This pathway is about 5.6 kcal/mol lower in energy than the first one.
The reaction concludes with a C-C (green) bond migration, induced by the chloride anion abstracting a hydrogen from the OH group. No such abstraction can occur as an inducement for the alternative (pink) bond to migrate, providing another explanation for the observed regiochemistry. The complete (concerted but asynchronous) reaction path can be seen in animated form below:
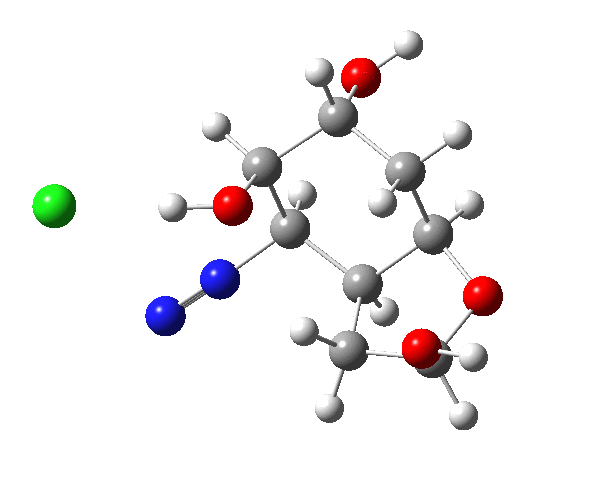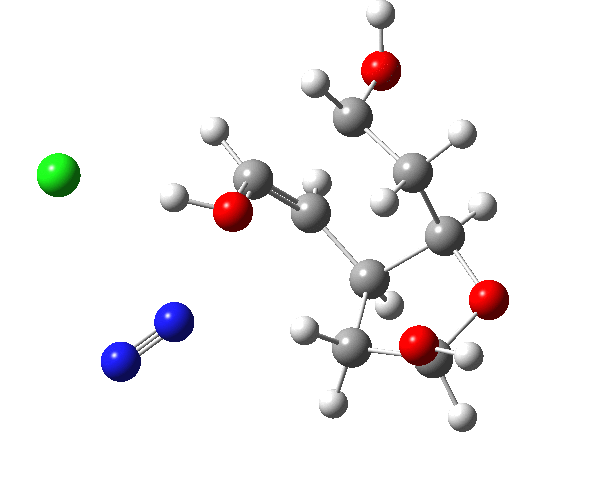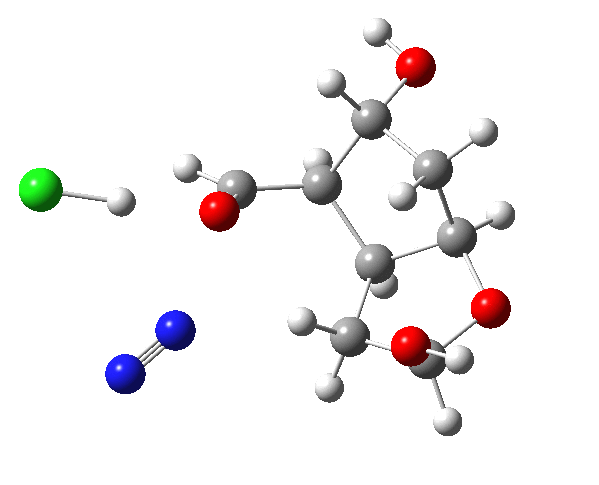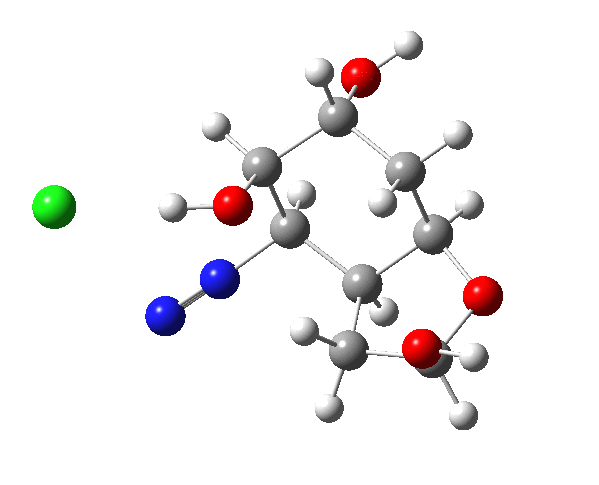
The plots below show the evolution of various properties of the system along the reaction path.
- Plot 1 of energy shows a small activation energy (7 kcal/mol), leading to an exothermic reaction by about 34 kcal/mol.
- The gradient plot 2 (the derivative of the energy with respect to the geometry) shows several interesting features
- The reaction starts at IRC = 1.5 with zero gradients.
- It reaches the transition state very early (IRC=0.0), at which point the gradients are again zero.
- and then the gradients (almost but not quite) reach zero again (IRC ~-2). This is called a hidden reaction intermediate and corresponds to the cations noted above (as an ion pair, with chloride anion). Because the ion pair has a large dipole moment, one might expect the reaction to be sensitive to the polarity of any solvent, and these hidden intermediates might become real ones in highly polar solvents.
- At IRC -5, the gradients become large as the carbon starts to migrate.
- The migration (with retention of stereochemistry, it is a cationic [1,2] sigmatropic shift) is induced by the chloride anion starting to abstract the proton from the OH group, in synchrony with the carbon migration.
- After IRC -8, we see only conformational changes occurring, which may also be interesting to analyse.
- Plot 3 shows the length of the breaking (migrating) C-C (green) bond
- Plot 4 the length of the newly forming (migrating) C-C bond. Note how initially, up to the transition state, the bond lengthens slightly, before slowly starting to contract at the transition state, but only does so sharply after IRC -3.
- Plots 5 and 6 show the length of the O...H and Cl...H bond as the proton transfer proceeds. This mostly occurs AFTER the transition state is passed, and so should not exhibit any kinetic isotope effect induced by e.g. deuterium substitution.
- Plot 7 shows the dipole moment evolving along the reaction. At the start the species is an ion pair (diazonium chloride), but as the reaction proceeds HCl is formed and the dipole moment decreases to that of a non-ionic compound.
The final product has the stereochemistry shown (atoms with halos in the model).
| Key species in the reaction | |
|---|---|
| Reactant (green and pink bonds) | |
| orbital overlap | orbital overlap |
| Favoured hidden intermediate carbonium ion: 0.0 | Disfavoured hidden intermediate carbonium ion: +5.6 |
| Transition state, doi: 9d8, IRC 9d9 | Product with stereochemistry |