Posts Tagged ‘chemical shift’
Friday, February 16th, 2018
Last year, this article[1] attracted a lot of attention as the first example of molecular helium in the form of Na2He. In fact, the helium in this species has a calculated‡ bond index of only 0.15 and it is better classified as a sodium electride with the ionisation induced by pressure and the presence of helium atoms. The helium is neither valent, nor indeed hypervalent (the meanings are in fact equivalent for this element). In a separate blog posted in 2013, I noted a cobalt carbonyl complex containing a hexacoordinate hydrogen in the form of hydride, H–. A comment appended to this blog insightfully asked about the isoelectronic complex containing He instead of H–. Here, rather belatedly, I respond to this comment!
(more…)
References
- X. Dong, A.R. Oganov, A.F. Goncharov, E. Stavrou, S. Lobanov, G. Saleh, G. Qian, Q. Zhu, C. Gatti, V.L. Deringer, R. Dronskowski, X. Zhou, V.B. Prakapenka, Z. Konôpková, I.A. Popov, A.I. Boldyrev, and H. Wang, "A stable compound of helium and sodium at high pressure", Nature Chemistry, vol. 9, pp. 440-445, 2017. https://doi.org/10.1038/nchem.2716
Tags:chemical bonding, Chemical elements, chemical shift, Chemistry, helium, Hydride, Hydrogen, Hypervalent molecule, Matter, Metal hydrides, Reducing agents, Transition metal hydride
Posted in Hypervalency | 4 Comments »
Saturday, January 13th, 2018
I discussed the molecule the molecule CH3F2- a while back. It was a very rare computed example of a system where the added two electrons populate the higher valence shells known as Rydberg orbitals as an alternative to populating the C-F antibonding σ-orbital to produce CH3– and F–. The net result was the creation of a weak C-F “hyperbond”, in which the C-F region has an inner conventional bond, with an outer “sheath” encircling the first bond. But this system very easily dissociates to CH3– and F– and is hardly a viable candidate for experimental detection. In an effort to “tune” this effect to see if a better candidate for such detection might be found, I tried CMe3F2-. Here is its story.
(more…)
Tags:Antibonding molecular orbital, candidate for experimental detection, chemical bonding, chemical shift, Chemistry, metal, Molecular orbital, Nature
Posted in Hypervalency | 2 Comments »
Monday, June 26th, 2017
About 18 months ago, there was much discussion on this blog about a system reported by Bob Pascal and co-workers containing a short H…H contact of ~1.5Å[1]. In this system, the hydrogens were both attached to Si as Si-H…H-Si and compressed together by rings. Now a new report[2] and commented upon by Steve Bachrach, claims a similar distance for hydrogens attached to carbon, i.e. C-H…H-C, but without the ring compression.
(more…)
References
- J. Zong, J.T. Mague, and R.A. Pascal, "Exceptional Steric Congestion in an <i>in</i>,<i>in</i>-Bis(hydrosilane)", Journal of the American Chemical Society, vol. 135, pp. 13235-13237, 2013. https://doi.org/10.1021/ja407398w
- S. Rösel, H. Quanz, C. Logemann, J. Becker, E. Mossou, L. Cañadillas-Delgado, E. Caldeweyher, S. Grimme, and P.R. Schreiner, "London Dispersion Enables the Shortest Intermolecular Hydrocarbon H···H Contact", Journal of the American Chemical Society, vol. 139, pp. 7428-7431, 2017. https://doi.org/10.1021/jacs.7b01879
Tags:10.1021, Blog, chemical shift, chemical shift difference, chemical shifts, gas phase, Oxygen, Steve Bachrach
Posted in Interesting chemistry | 3 Comments »
Tuesday, May 23rd, 2017
This is taking place in the idyllic surroundings of the Niederwald forest, Rüdesheim, Germany. Here I highlight only aspects of the first three talks.
(more…)
Tags:article processing charges, Bad Kreuznach, chemical shift, chemical terms, City: Rüdesheim, Country: Germany, Hesse, Hesse-Nassau, Ian Bruno, Jeremy Frey, Klaus Tochtermann, Leah McEwen, Martin Hicks, metadata tools, Niederwald, Niederwalddenkmal, Quotation, Rüdesheim, Rüdesheim am Rhein, Rüdesheim an der Nahe, Rheingau-Taunus-Kreis, Rhine, Richard Kidd, spectroscopy, States of Germany, Stuart Chalk, Technology/Internet
Posted in Chemical IT | 1 Comment »
Tuesday, August 12th, 2014
One thing leads to another. Thus in the previous post, I described a thermal pericyclic reaction that appears to exhibit two transition states resulting in two different stereochemical outcomes. I noted that another such reaction appeared to be a [1,6] carousel migration in homotropylium cation,[1] where transition states for both retention and inversion of the configuration of the migrating group (respectively formally allowed and forbidden) were reported (scheme below). Here I explore this system further.  Firstly, the pathway leading to inversion.[2] The reaction path (ωB97XD/6-311G(d,p)/SCRF=chloroform) has got a very odd (table-top mountain) shape, whereby the region of the transition state (IRC = 0.0) is very flat, and the region close to reactant and (identical) product is very steep. The gradient norm shows this best, with sharp spikes at IRC ± 4.2. Something clearly is happening here to cause this behaviour. Before moving on to analyze this, I want you first to observe the methyl groups below. Note how one of them rotates at the start of the process, and the other at the end. I have elsewhere called this behaviour the methyl flag, and it is due to stereoelectronic re-alignments of the C-H groups accompanying the changes in the conjugated array.
Firstly, the pathway leading to inversion.[2] The reaction path (ωB97XD/6-311G(d,p)/SCRF=chloroform) has got a very odd (table-top mountain) shape, whereby the region of the transition state (IRC = 0.0) is very flat, and the region close to reactant and (identical) product is very steep. The gradient norm shows this best, with sharp spikes at IRC ± 4.2. Something clearly is happening here to cause this behaviour. Before moving on to analyze this, I want you first to observe the methyl groups below. Note how one of them rotates at the start of the process, and the other at the end. I have elsewhere called this behaviour the methyl flag, and it is due to stereoelectronic re-alignments of the C-H groups accompanying the changes in the conjugated array. 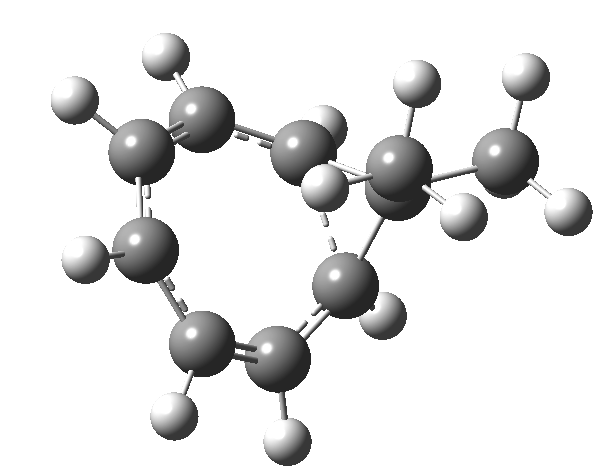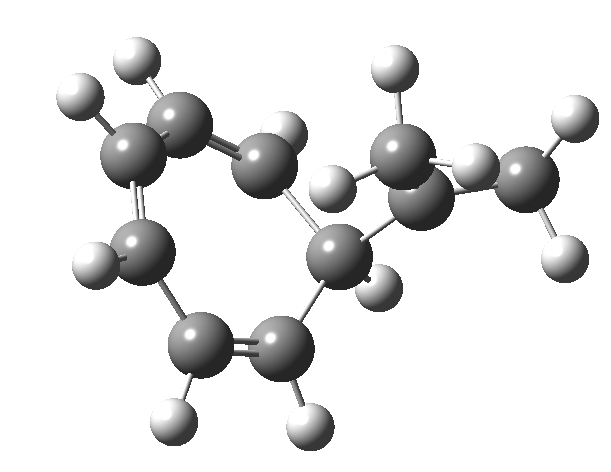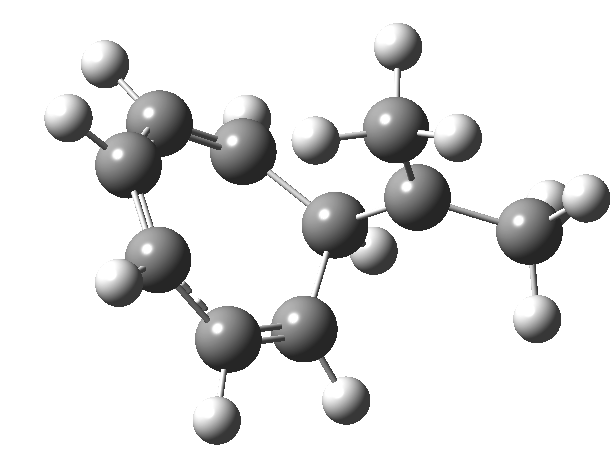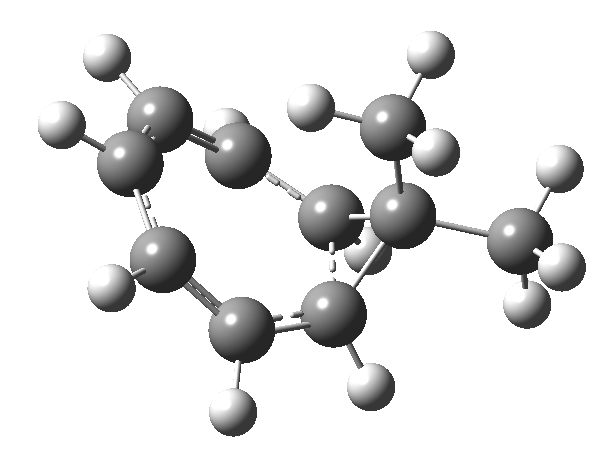

 The homotropylium cation is said to be homoaromatic, indicating that cyclic conjugation can be maintained across a ring in which the σ framework is interrupted at one point. A NICS probe placed at the ring critical point of this molecule reveals a chemical shift of -11.3 ppm[3], very similar to eg that obtained for benzene itself. The three highest doubly occupied NBOs (below) show two normal π-type orbitals and one rather different one that spans the homo-bond (the MOs, before you ask, are a bit of a mess, with lots of mixed contributions from other parts of the σ framework).
The homotropylium cation is said to be homoaromatic, indicating that cyclic conjugation can be maintained across a ring in which the σ framework is interrupted at one point. A NICS probe placed at the ring critical point of this molecule reveals a chemical shift of -11.3 ppm[3], very similar to eg that obtained for benzene itself. The three highest doubly occupied NBOs (below) show two normal π-type orbitals and one rather different one that spans the homo-bond (the MOs, before you ask, are a bit of a mess, with lots of mixed contributions from other parts of the σ framework).
(more…)
References
- A.M. Genaev, G.E. Sal’nikov, and V.G. Shubin, "Energy barriers to carousel rearrangements of carbocations: Quantum-chemical calculations vs. experiment", Russian Journal of Organic Chemistry, vol. 43, pp. 1134-1138, 2007. https://doi.org/10.1134/s1070428007080076
- H.S. Rzepa, "Gaussian Job Archive for C10H13(1+)", 2014. https://doi.org/10.6084/m9.figshare.1134556
- H.S. Rzepa, "Gaussian Job Archive for C10H13(1+)", 2014. https://doi.org/10.6084/m9.figshare.1135694
Tags:chemical shift, higher energy, Sangean Table Top Portable Audio Device
Posted in pericyclic, reaction mechanism | 5 Comments »
Monday, July 8th, 2013
A feature of a blog which is quite different from a journal article is how rapidly a topic might evolve. Thus I started a few days ago with the theme of dicarbon (C2), identifying a metal carbide that showed C2 as a ligand, but which also entrapped a single carbon in hexa-coordinated mode. A comment was posted bringing attention to the origins of the discovery of hexacoordinated carbon, and we moved on to exploring the valency in one such species (CLi6). Here I ask if hydrogen itself might exhibit such coordination.
(more…)
Tags:chemical shift, metal atoms, metal carbide
Posted in Hypervalency, Interesting chemistry | 2 Comments »
