The previous post talked about making links or connections. And part of the purpose for presenting this chemistry as a blog is to expose how these connections are made, or or less as it happens in real time (and not the chronologically sanitized version of discovery that most research papers are). So each post represents an evolution or mutation from the previous one. To recapitulate, we have seen how the idea of cyclopentadienyl anion as a ligand for a dipositive carbon atom has evolved. Let us move in yet another direction; the cyclobutadienyl dianion. This ligand has recently been shown to bind Mg2+ (DOI: 10.1002/ejic.200800066), so why not He2+? And picking up again the previous theme, we will then protonate the bound complex. The result now is a monocation, and it has the C4v-symmetric structure shown below (DOI: 10042/to-2438). This bears some resemblance to pyramidane, a neutral C5H4 compound with hemispherical carbon reported in 2001 (DOI: 10.1021/jp011642r) which is also a stable minimum in the potential energy surface.

C4-symmetric pentavalent carbon
Now, the apical C-C bonds have shrunk to 1.58Å, the trampoline mode is increased to 970 cm-1 and the apical C-H frequency to 3291 cm-1. The apical C-C value for the AIM bond critical point ρ(r) is up 0.195 au and the disynaptic basin integration in that region is now 1.1 electrons. Replacing the apical C-H by C-F further strengthens the system (DOI: 10042/to-2447); the apical C-C bonds contract slightly to 1.57Å, the bouncing castle/trampoline mode shoots up to ν 1595 cm-1 , ρ(r) reaches 0.201 au and the disynaptic basins 1.25 electrons. With this latter system, the C-F disynaptic basin contains only 1.08 electrons, suggesting it is similar in nature to the other four bonds surrounding the apical carbon, i.e. this carbon is surrounded by five more or less equivalent bonds. The pseudo-halogen CN can also replace the F (DOI: 10042/to-2449) to similar effect (ρ(r)C-C 0.190, ρ(r)C-CN 0.290).
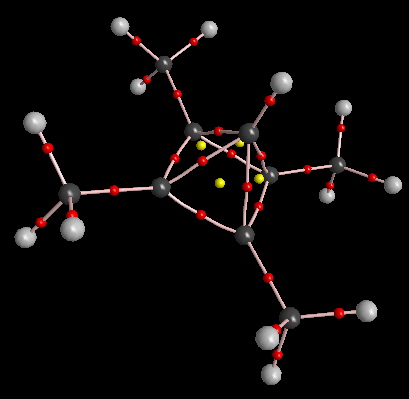 AIM Analysis |
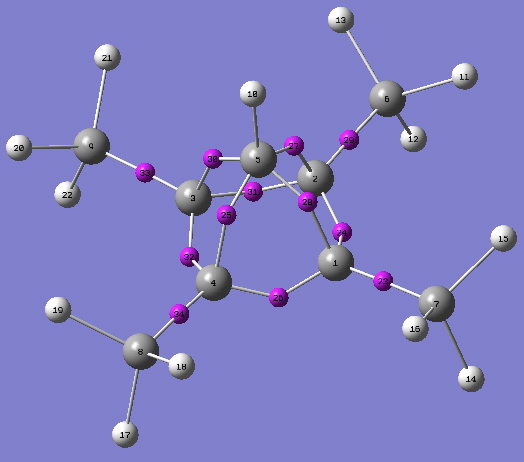 ELF Basin centroids. Click for 3D |
We are back to pentavalent, pentacoordinate carbon again, but we have gradually optimized the properties of the system for five short C-C bonds surrounding one carbon atom, and the largest electron density and disynaptic basin integration. Whilst the sentiments expressed by Hoffmann, Schleyer and Schaefer (DOI: 10.1002/anie.200801206) for more realism in predicting molecules must not be ignored, it is to be hoped that the original suggestions made here will lead to the discovery of realistic and makeable molecules exhibiting true C-C hypervalency.
Tags: Hypervalency, Interesting chemistry, potential energy surface, pseudo