All organic chemists are familiar with the stereochemical notation for bonds, as shown below. But I had difficulty tracking down when it was introduced, and by whom. I offer a suggestion here, but if anyone reading this blog has got a better/earlier attribution, please let us know!
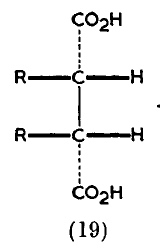
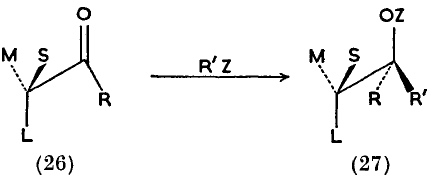
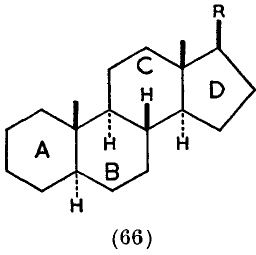
I suggest that the source is an article written by Derek Barton and R. C. Cookson in 1955 and published in 1956, entitled “The principles of conformational analysis” (DOI: http://dx.doi.org/10.1039/QR9561000044 ). Some examples are shown below. Compound 19 makes explicit the Fischer convention; 26/27 are indeed very modern, and 66 uses not wedges but bold bonds (which is very common nowadays but suffers from having a slightly different semantic interpretation which was proposed by Maehr).
One might ask what another master of the period, R. B. Woodward was using. Thus in his 1956 article on the synthesis of lysergic acid, we see this. Plenty of stereochemistry, but not annotated as per above! What you do find (and with Barton as well) is essentially modern use of the arrow pushing conventions, so by this period it was thoroughly established.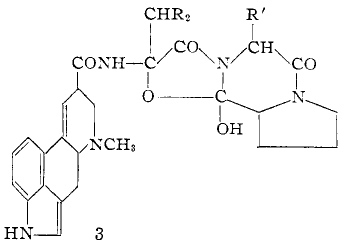
Going back to 1951, we see Stork offering a stereospecific synthesis (as far as I can tell, the first use of precisely this term in the literature). But in this example, there is no real need for clarification using the modern stereochemical notation.
So, can anyone find examples of modern notation earlier than Barton’s usage?
Tags: Derek Barton, Historical, R. C. Cookson
Henry,
I have found that with questions about historical facts in chemistry, there are a few incredibly knowledgeable historians of chemistry, among them Prof. William B. Jensen, just retired from the Univ. of Cincinnati.
He has a regular column in the J. Chem. Educ. entitled “Ask a historian”. Those contributions he has collected in a PDF file and you can download them (24 MB) from here
As it happens, he has just written a column entitled “Origin of Stereochemical Line and Wedge Symbols” (page 109). It says at the end that the text has been submitted to the J. Chem. Educ. in 2012, but I have not yet seen it online.
The funny thing is, he also cites a source from 1956 (Cram and Hammond) as the first appearance in print, but a different one. Maybe you want to inform Dr. Jensen about your finding. In addition he shows earlier examples of wedges dating back to the 1930s.
Addendum: Dotted lines and thick lines used in combination already by R. Kuhn in 1932, see Jensens article.
Thanks Lukas. Perhaps the surprising aspect is that it has taken until 2012 to write a published history of this aspect of notation. And that (apart from CIP), the originators of notation rarely get full recognition. Even by 1956, the use of this now ubiquitous stereochemical notation was not common; thus Woodward appears not to have endorsed it.
Since the reference you give is a large download, I have taken the liberty of quoting the figure you refer to here:
A digression, but the diagram captioned as by Wells is presumably of diborane, the bridging structure having been famously suggested in 1943 by Longuet-Higgins and Bell (DOI: 10.1039/JR9430000250), but not illustrated as clearly as by Wells. But the oddity is why represent the boron atoms as being so much smaller than the hydrogen? I do not have immediate access to the article referred to (A. F. Wells, Structural Inorganic Chemistry, Clarendon, Oxford, 1945).