In my previous post I speculated why bis(trifluoromethyl) ketone tends to fully form a hydrate when dissolved in water, but acetone does not. Here I turn to asking why formaldehyde is also 80% converted to methanediol in water? Could it be that again, the diol is somehow preferentially stabilised compared to the carbonyl precursor and if so, why?
Methanediol.
The lowest energy geometry is shown above. Conspicuously, it does not form an intramolecular O-H…O hydrogen bond, but adopts a C2-symmetric form. NBO analysis for this geometry reveals two interactions larger than the rest. The first, shown below, involves overlap of an oxygen lone pair (Lp) donor orbital with a C-H acceptor (purple+blue, orange-red), and this is worth E(2) 6.1 kcal/mol (there are two of these). Unfortunately, the analogous NBO interaction in acetone itself originating from a C-Me bond as acceptor is 6.3 kcal/mol and so this interaction does not differentiate between the two.
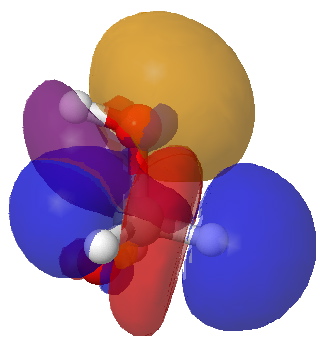
NBO interaction between O Lp and a C-H acceptor. Click for 3D
The larger NBO interaction of E(2) = 16.9 kcal/mol arises from the same donor orbital interacting with the C-O acceptor (the presence of the more electronegative oxygen accounts for it being the better acceptor). In acetone however, this too has the high value of 16.8 kcal/mol.
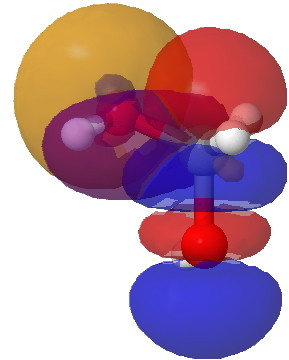
NBO interaction between Oxygen Lp and C-O acceptor. Click for 3D.
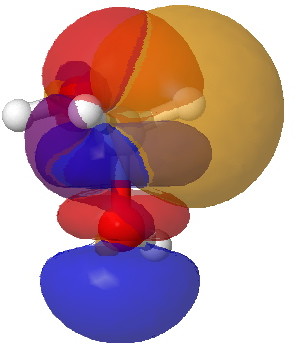
interaction between a C-H donor and a C-O acceptor. Click for 3D
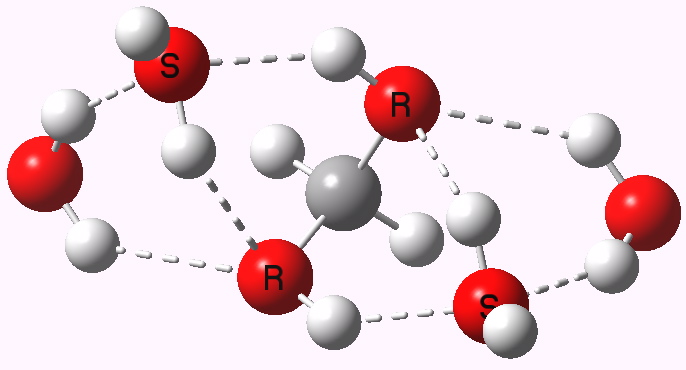 Methanediol with four water molecules. Click for 3D. |
The water molecules combine with the methanediol to form an elegant lattice of hydrogen bonds, involving two rings of three oxygens and one ring of four oxygens. This compact motif is less stable for propanediol, which instead prefers a structure forming fewer hydrogen bonds, largely because of the presence of the hydrophobic methyl groups. The result is that the free energy of hydration of formaldehyde to the diol, assisted by hydrogen bonds formed to four water molecules, is exothermic at -1.2 kcal/mol, whereas that for acetone is endothermic at +7.5 kcal/mol.
As with most things water, a proper stochastic exploration of all the possible configurations of the hydrogen bonds is necessary for a definitive explanation. But it does seem that a probable theory for why formaldehyde readily forms a diol whereas acetone does not lies not so much in stereoelectronic donor-acceptor interactions but in the hydrogen bonds set up in the solvated diol.
Tags: free energy, lowest energy geometry, O Lp, Oxygen Lp, solvation, Tutorial material
Thanks for another insightful post. The important contributions of the hydration of the product is convincing. This could also answer, why formaldehyde is hydrated to >99.9% in solution (see comment below), but still it is impossible to isolate the hydrate CH2(OH)2 in pure form (evaporation produces CH2O + H2O). The stability of the hydrate is connected to the hydration shell!
Henry, there is a mistake in Wikipedia. An aqueous solution of formaldehyde is not 80% hydrated, but 99.9% or higher. This follows form K(hydr) = [hydrate]/[carbonyl] = 2000 , where the concentration of water (ca 55 mol/L) is already included in K(hydr).This is not just theory. NMR spectra of formaldehyde solutions show no remaining peak for the carbonyl component. This was for example noted in a study on the hydrolysis of dimethoxymethane, where the hydrate was the only component analyzed for the product:
http://pubs.acs.org/doi/pdf/10.1021/ja00376a040
Or also in this study determining the pKa of dihydroxymethane:
http://pubs.rsc.org/en/Content/ArticleLanding/1998/JC/a706993f
It is possible that the actual content of fromaldehyde hydrate is somewhat lower than calculated at high formaldehyde concentration; not because of free carbonyl content, but due to oligomer formation (polyoxymethylene). However, I don’t think the 80% number mentioned in Wikipedia is correct. The number is certainly not from the Anslyn/Doubherty book cited for K(hydr).
This is extremely helpful as i’ve been conducting some experiments to look at the “crossling linking” effect on globular peptide polymers and monomeric peptides using some mass spec devices and have spent many an hour arguing with colleagues over this topic. I can see where wiki got the 80% as when working out the theoretical kinetic equilibriums between HCHO and HCH(OH)2, taking HCHO as ~3pKa and water as ~7, we get a KEq of 0.8 assuming that the product being HCH(OH)2 has a pKa of 5.
Now takiing these 2 estimated PKa’s of HCHO being 3 and HCH(OH)2 as 5 and for the purpose of this we’ll just look at lysine sidechain with 10.5 Pka, theoreticallly the condensation reactions are HCHO + Lys with a 1.11 Keq while HCH(OH)2 + Lys is 0.71Keq, sugesting that dehydrated form may be required for this reaction to take place This is only for the first part of the reaction as this will then need to stabilise wiith another amine, imine or aromatic ring (mannich reaction) forming a 2 covalent bond bridge either within the same peptide or from another peptide in close proximity, again the carbon atom from the second reaction may or may not need to be hydrated, or even dehydrated if H2O is not liberated from the first reaction.
any thoughts?
Jason, the pKa are irrelevant, because at neutral pH none of the species present in aqueous formaldehyde is ionized to any particular degree. In fact, the measured pKa of CH2(OH)2 is 13.3, which is somewhat lower than a typical alcohol, as expected by the sigma acceptor effect excerted by the second OH group.
In the meantime I found out where the 80% are coming from: indeed, in aqueous solutions of formaldehyde, there is an oligomerization equilibrium between CH2(OH)2 and HO-(CH2-O)n-H with n = 2, 3, 4, 5, and so on.
In a 3% solution of formaldehyde in water, there is 84.5% monomer (methylene glycol), 14.5% dimer, and 1% trimer. Note that the concentration of non-hydrated formaldehyde is too small to measure, as expected from K(hydrat) = 2200.
In a 5% solution, there should be pretty close to 80% monomer and correspondingly more dimer, trimer etc. This can be seen from the data (Table V and Figure 5) in this paper:
http://pubs.acs.org/doi/abs/10.1021/ac00254a041
So, indeed formaldehyde is present to 80% as methylene glycol in 5% aqueous formaldehyde, but this does not mean that the remaining 20% is non-hydrated; in fact, the non-hydrated form is present to less than 0.1%