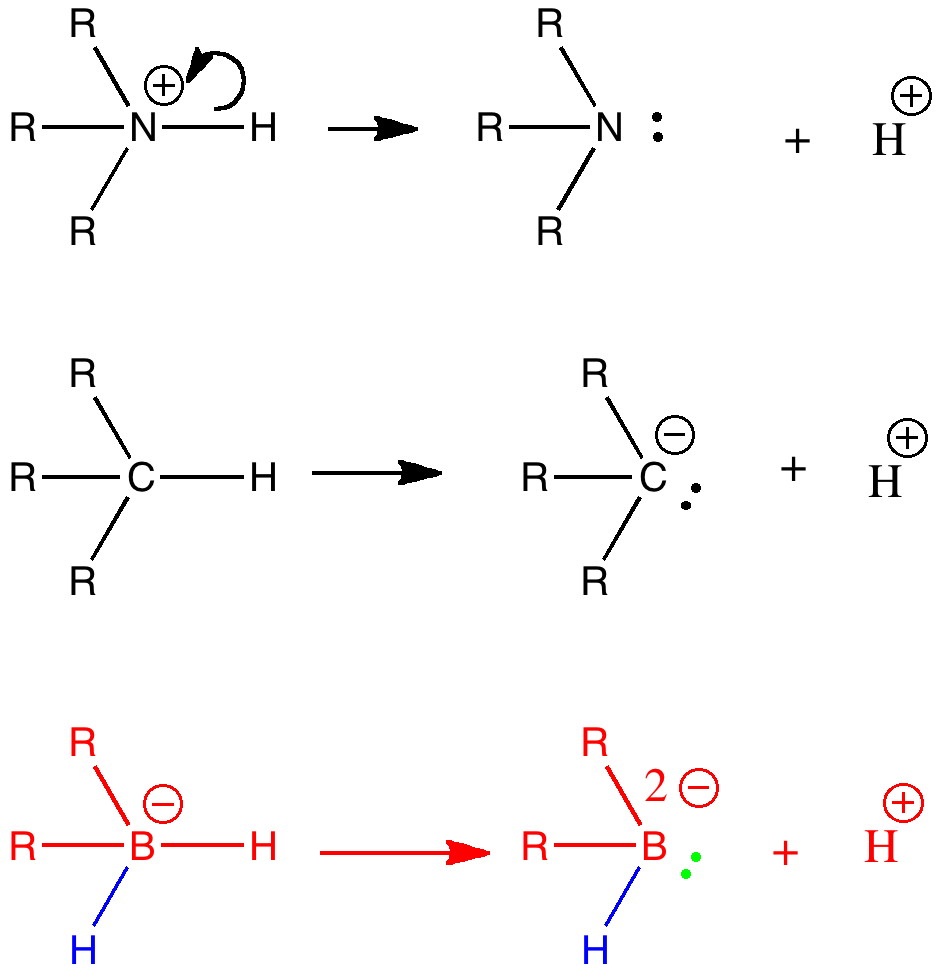
I have often heard the question posed “how much of chemistry has been discovered?” Another might be “has most of chemistry, like low-hanging fruit, already been picked?“. Well, time and time again, one comes across examples which are only a simple diagram or so away from what might be found in any introductory chemistry text, and which would tend to indicate the answers to these questions is a resounding no. Take for example the three reactions shown below.
The top row shows a quaternary ammonium salt behaving as an acid in loosing a proton to become an amine. The shared covalent N-H bond becomes a lone pair on the amine, concepts first introduced by G. N. Lewis almost 100 years ago, and still an absolute bedrock of introductory chemistry. A little later, students learn that hydrocarbons are much less acidic. The conjugate base, a carbanion, is nevertheless a species very well known to synthetic chemists. You will also have noticed the iso-electronic progression in moving from nitrogen to carbon. Well, it’s a tiny lateral extrapolation to ask what might happen to boron, the next element in this iso-electronic series? The answer is shown as the bottom reaction (red) in an article recently published by Bertrand and co-workers (DOI: 10.1126/science.1207573).
Why is this so newsworthy? Because in the Lewis model, boron has hitherto always acted in its known chemistry as a recipient of a lone pair, but never potentially as a donor! It is true however, that e.g. a borohydride anion (BH4–) can donate not so much a lone pair as the covalent pair in one of the B-H bonds, in effect a hydride anion. So the fascinating species made by Bertrand and co-workers, R2HB2-and named a borylene now raises a new question. What does the borylene shown above prefer to do, donate a lone pair (green above) or instead donate a B-H covalent electron pair (blue above)? Does one enhance the other? Extrapolating introductory textbook chemistry can so easily lead to new chemistry, so don’t give up hope of ever doing it yourself!
I noted that hydrocarbons are very much less acidic than ammonium salts (n-butyl lithium, a typical unstabilized carbanion, is a very dangerous beast, although benzyl anion does form stable crystals). One might imagine that an unstabilized borylene would be even more so. In fact, it was created as a (double) zwitterion, with the two R groups themselves each bearing a +ve charge to balance the two -ve charges on the boron. It is shown below, surrounded by inert alkyl groups to prevent it reacting.
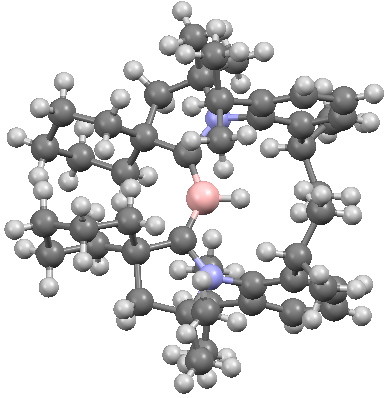
A crystalline borylene. Click for 3D.
Postscript. The HOMO referred to above can be seen as a 3D model if you click on the graphic below. Next to it is the HOMO-2 orbital, which has B-H character, showing that both types of reactivity could be present.
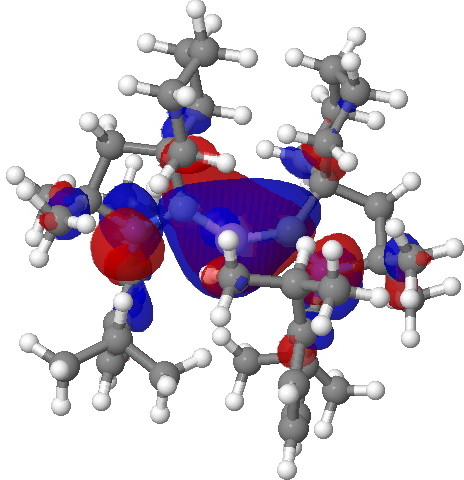 HOMO molecular orbital for the borylene. Click for 3D. |
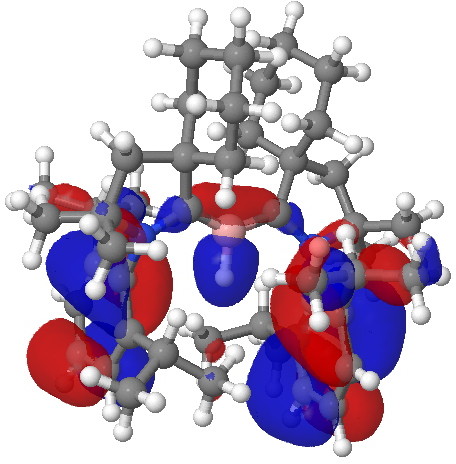 HOMO-2 orbital, showing B-H donating character. Click for 3D. |
Tags: basic boron, Tutorial material