An article with the title shown above in part recently appeared.[1] Given the apparent similarity of HF1- to CH3F1- and CH3F2-, the latter of which I introduced on this blog previously, I thought it of interest to apply my analysis to HF1-.
The authors[1] conclude that “the F atom of HF− is negative and hypervalent and the bonding is more covalent than ionic“. So, firstly an NBO analysis. Shown below is the singly occupied NBO (ωB97XD/Def2-TZVPPD calculation, FAIR data DOI: 10.14469/hpc/3274)
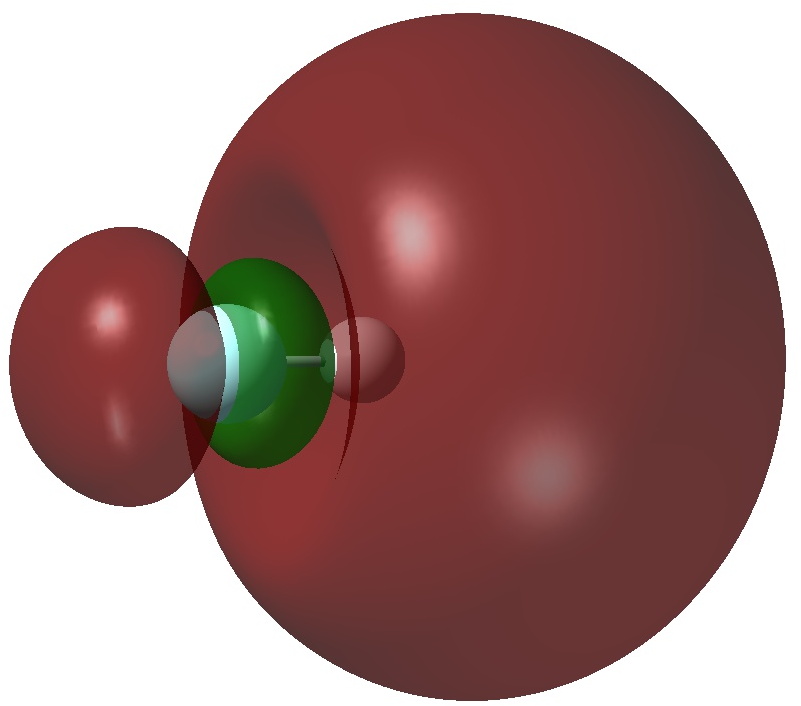
The nature of this orbital is that most of it is located beyond the hydrogen and a node is apparent between the F and H (it is H-F antibonding). The Wiberg bond index for both H and F is 0.48, as is the F-H bond order. This matches with the observation of one electron in an orbital which is H-F antibonding. NBO analysis also indicates that the atomic orbital contributions to F[core]2S(1.93)2p(5.77)3S(0.05)3p(0.04)3d(0.01) and H 1S(0.96)2S(0.12)2p(0.10) show modest Rydberg character on the hydrogen, less on the fluorine. By this criterion, it is the hydrogen and not the fluorine that is hypervalent!
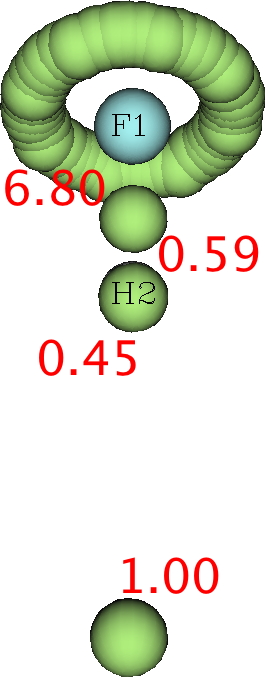
Next, the ELF analysis (respectively FAIR data DOI: DFT 10.14469/hpc/3377 and CASSCF(7,8) 10.14469/hpc/3394).
| DFT | CASSCF(7,8) |
|---|---|
 |
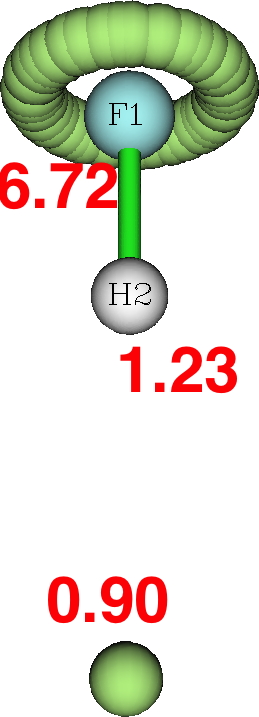 |
Both these methods reveal a monosynaptic ELF basin located away from the H, with an integration of about 1 electron. The F lone pairs form a torus around the F and the total of electrons around the fluorine is <8. So again no evidence from ELF that the fluorine is hypervalent.
In fact this analysis resembles one feature of CLi6. With nominally 12e apparently contributing to the shared C-Li shell, CLi6 was described as hypervalent.[2] In fact ~3e of these are “expelled” from the shared C-Li regions into Li-Li regions, where they contribute only to lithium valency and not to the carbon valency. With HF1-, the additional electron apparently responsible for the hypervalency contributes only to the H, but not to the F valencies. With CH3F2-, the two injected electrons do appear to contribute to the C-F bond, making it a true hyperbond.
So based on the above, I cannot entirely agree with the assertion that “the F atom of HF− is negative and hypervalent”[1], but I might suggest that something more unusual is happening, the hydrogen is (mildly) hypervalent!
References
- M. Liu, H. Chen, C. Chin, T. Huang, Y. Chen, and Y. Wu, "Identification of a Simplest Hypervalent Hydrogen Fluoride Anion in Solid Argon", Scientific Reports, vol. 7, 2017. https://doi.org/10.1038/s41598-017-02687-z
- H. Kudo, "Observation of hypervalent CLi6 by Knudsen-effusion mass spectrometry", Nature, vol. 355, pp. 432-434, 1992. https://doi.org/10.1038/355432a0