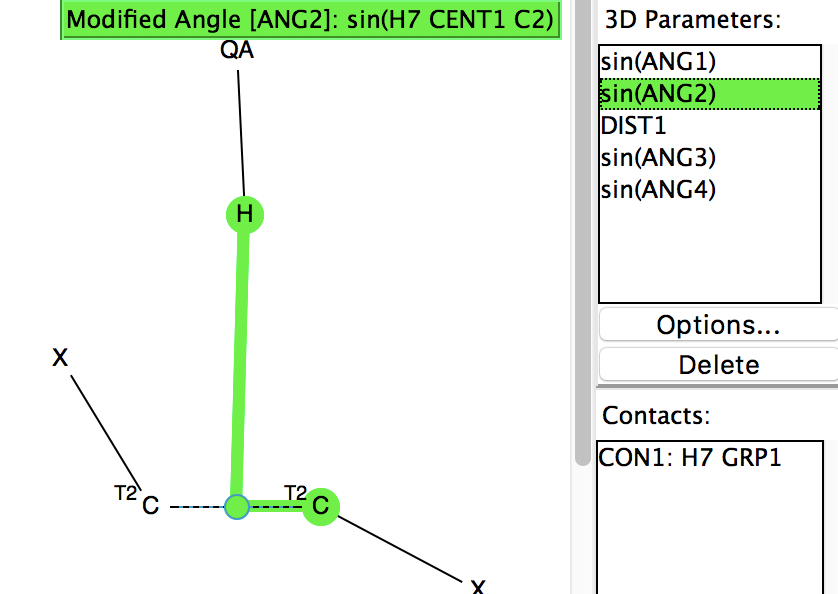
Following on from my re-investigation of close hydrogen bonding contacts to the π-face of alkenes, here now is an updated scan for H-bonds to alkynes. The search query (dataDOI: 10.14469/hpc/2478) is similar to the previous one:
- QA is any of N,O,F,Cl.
- X is any atom, including metals and non-metals.
- The carbon atoms are both specified as 2-coordinate, and the C-C bond type as any.
- The distance is from the hydrogen (normalised) to the C-C centroid, restricted to < 2.5Å to capture just the shortest examples.
- The mean of the sines of the two angles subtended at the centroid is calculated to indicate whether the approach is orthogonal.
- The mean of the absolute value of the sines of the two angles subtended at each carbon is calculated to indicate how non-linear the X-C-C angle is.
- Other constraints are no disorder, no errors and R < 0.05.
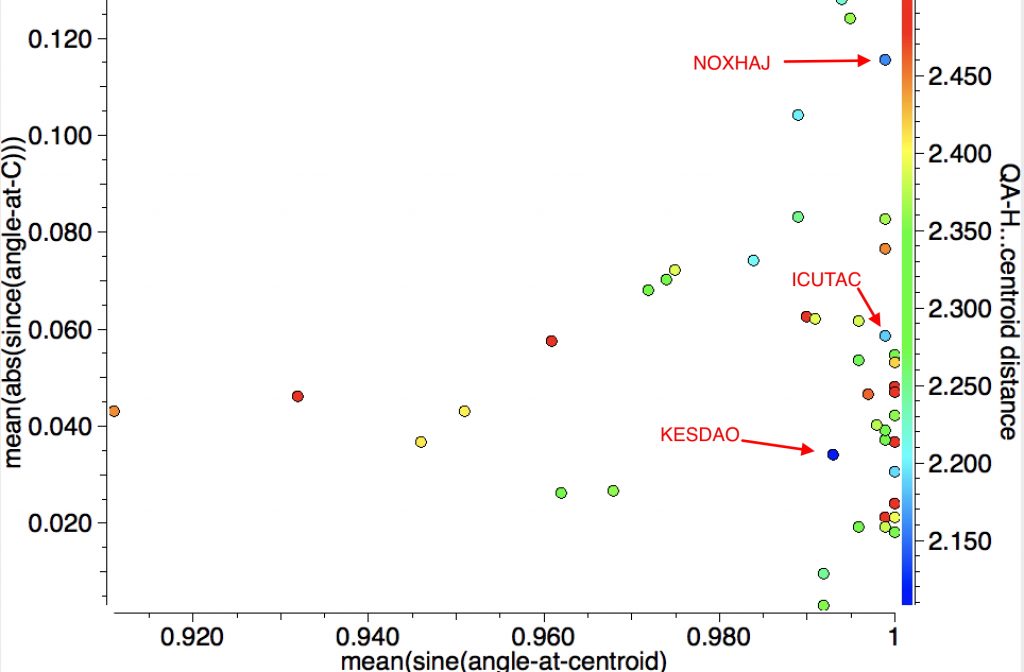
First the intermolecular hits (38). Prominent short examples include:
| Entry | Article DOI | DataDOI | H-centroid distance |
|---|---|---|---|
| NOXHAJ | [1] | ‡ | 2.16Å |
| KESDAO | [2]† | 10.5517/CC9YHJ7† | 2.11Å |
| ICUTAC | [3] | 10.5517/CC9PNSD | 2.19Å |
 |
|||
In most of the stronger examples (blue), the approach of the hydrogen is perpendicular to the C-C bond centroid (X-axis of plot above). Many however exhibit significant bending (Y-axis of plot above) from linearity at the two carbons (~173°), mostly away from the H but in some examples towards the H!
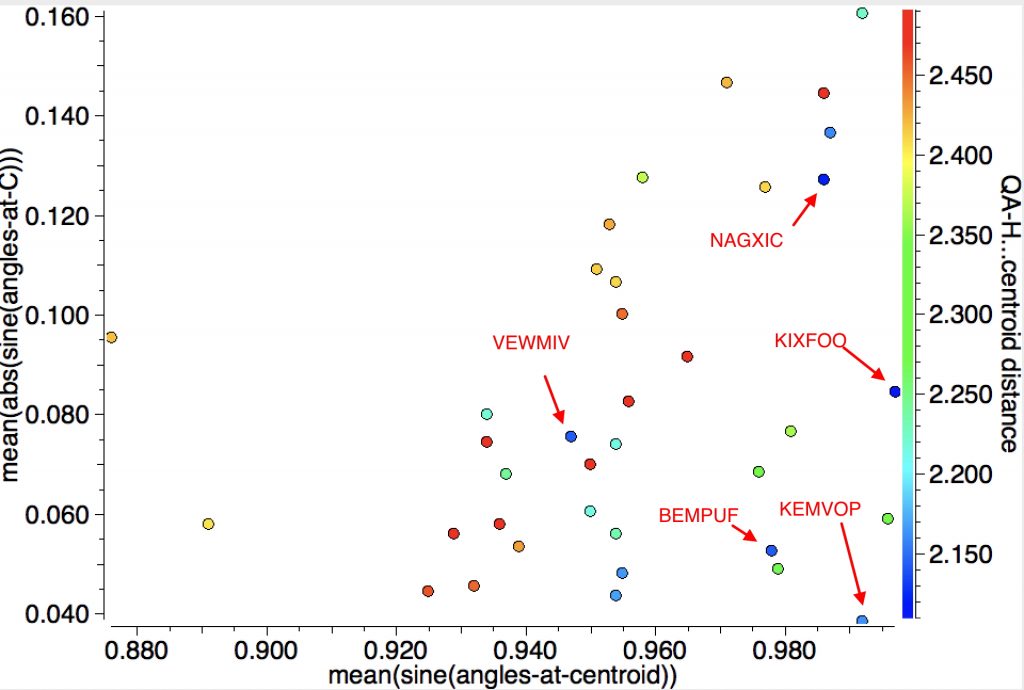
Selected entries from the intra-molecular search (34 hits) are shown below. Perhaps due to the intra-molecular nature, the angle of approach of the H is more variable than the intermolecular examples and the bending of the erstwhile X-C-C angle is again prominent.
| Entry | Article DOI | H-centroid distance | |
|---|---|---|---|
| KIXFOO | [4] | 2.11Å | |
| KEMVOP | [5] | 2.16Å | |
| BEMPUF | [6] | 2.14Å | |
| VEWMIV | [7] | 2.14Å | |
 |
|||
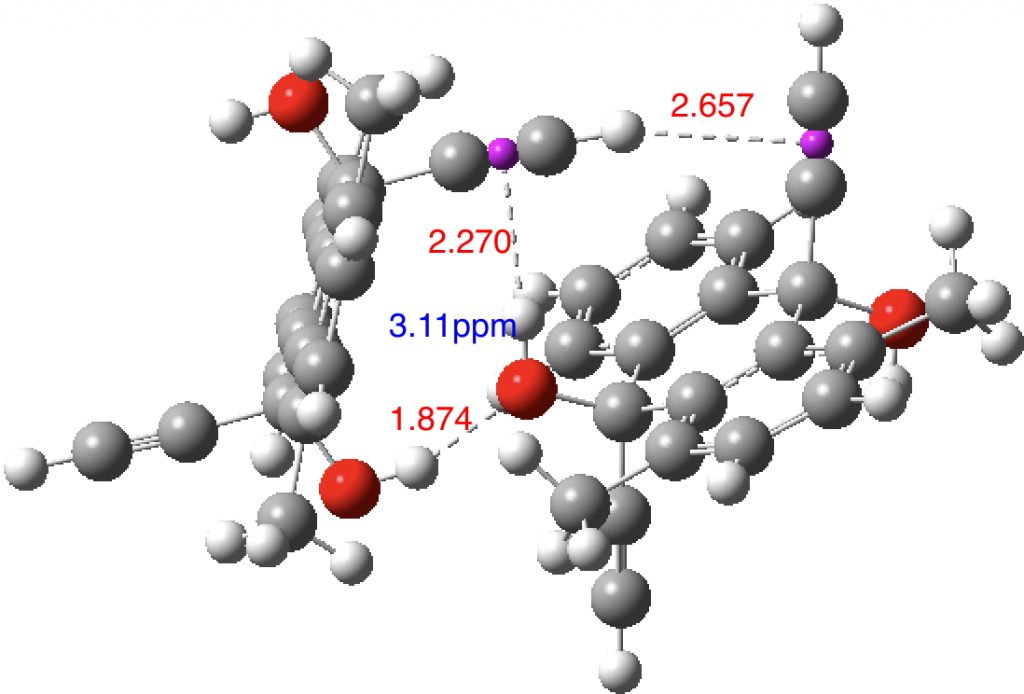
ωB97XD/Def2-TZVPP calculations of one intermolecular example, ICUTAC (two molecules, dataDOI: 10.14469/hpc/2482) and one intramolecular case, KIXFOO (dataDOI: 10.14469/hpc/2481). For the former, crystal packing compressions perhaps provide some shortening of the hydrogen bond and the molecule also includes an example of a short C-H to π interaction (obs[3] 2.63Å).
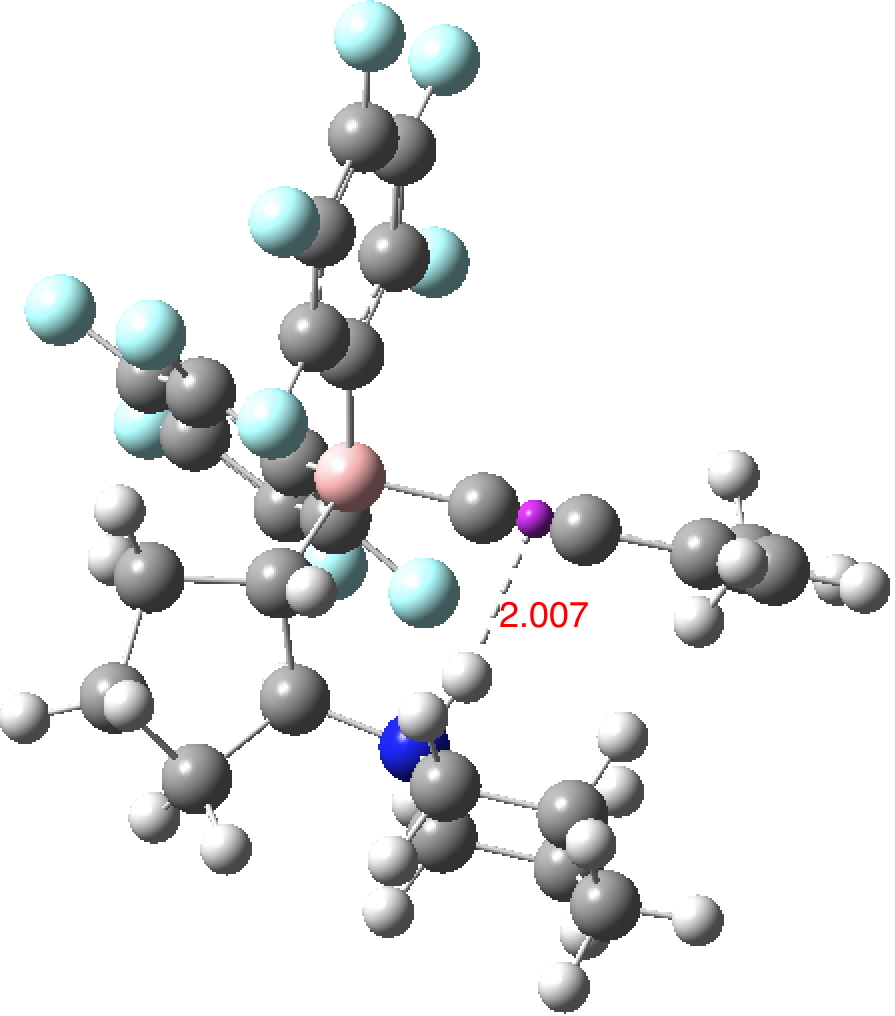
What is noticeable from reading the abstracts of the articles cited above is that these hydrogen bonds are rarely commented upon by the authors and it does seem that most of these close contacts are serendipitous (they were not designed). All are somewhat longer than the shortest distances encountered for alkenes and it would be interesting to establish if this is an intrinsic property of the triple bond or whether less effort has hitherto been expended on designing closer approaches.
‡ Not all entries have an assigned dataDOI at CCDC.
†CrossRef DOIs here are collected as a citation at the bottom of the post using the WordPress KCite plugin. Unfortunately for a few months now, this plugin has stopped recognising DataCite DOIs, which is why here they are treated differently from CrossRef DOIs. This is purely a current attribute of the KCite plugin and does not imply any fundamental difference in the two types of DOI, other than one tends to be used as persistent identifiers of journal articles and the other of datasets.
References
- M. Akita, M. Chung, A. Sakurai, S. Sugimoto, M. Terada, M. Tanaka, and Y. Moro-oka, "Synthesis and Structure Determination of the Linear Conjugated Polyynyl and Polyynediyl Iron Complexes Fp*−(C⋮C)<i><sub>n</sub></i>−X (X = H (<i>n</i>= 1, 2); X = Fp* (<i>n</i>= 1, 2, 4); Fp* = (η<sup>5</sup>-C<sub>5</sub>Me<sub>5</sub>)Fe(CO)<sub>2</sub>)<sup>1</sup>", Organometallics, vol. 16, pp. 4882-4888, 1997. https://doi.org/10.1021/om970538m
- J. Forniés, S. Fuertes, A. Martín, V. Sicilia, E. Lalinde, and M.T. Moreno, "Homo‐ and Heteropolynuclear Platinum Complexes Stabilized by Dimethylpyrazolato and Alkynyl Bridging Ligands: Synthesis, Structures, and Luminescence", Chemistry – A European Journal, vol. 12, pp. 8253-8266, 2006. https://doi.org/10.1002/chem.200600139
- R. Banerjee, R. Mondal, J.A.K. Howard, and G.R. Desiraju, "Synthon Robustness and Solid-State Architecture in Substituted <i>g</i><i>em</i>-Alkynols", Crystal Growth & Design, vol. 6, pp. 999-1009, 2006. https://doi.org/10.1021/cg050598s
- B. Xu, K. Bussmann, R. Fröhlich, C.G. Daniliuc, J.G. Brandenburg, S. Grimme, G. Kehr, and G. Erker, "An Enamine/HB(C<sub>6</sub>F<sub>5</sub>)<sub>2</sub> Adduct as a Dormant State in Frustrated Lewis Pair Chemistry", Organometallics, vol. 32, pp. 6745-6752, 2013. https://doi.org/10.1021/om4004225
- M.J. Pouy, S.A. Delp, J. Uddin, V.M. Ramdeen, N.A. Cochrane, G.C. Fortman, T.B. Gunnoe, T.R. Cundari, M. Sabat, and W.H. Myers, "Intramolecular Hydroalkoxylation and Hydroamination of Alkynes Catalyzed by Cu(I) Complexes Supported by <i>N</i>-Heterocyclic Carbene Ligands", ACS Catalysis, vol. 2, pp. 2182-2193, 2012. https://doi.org/10.1021/cs300544w
- R.D. Dewhurst, A.F. Hill, and M.K. Smith, "Heterobimetallic C<sub>3</sub> Complexes through Silylpropargylidyne Desilylation", Angewandte Chemie International Edition, vol. 43, pp. 476-478, 2004. https://doi.org/10.1002/anie.200352693
- T. Holtrichter-Rößmann, C. Rösener, J. Hellmann, W. Uhl, E. Würthwein, R. Fröhlich, and B. Wibbeling, "Generation of Weakly Bound Al–N Lewis Pairs by Hydroalumination of Ynamines and the Activation of Small Molecules: Phenylethyne and Dicyclohexylcarbodiimide", Organometallics, vol. 31, pp. 3272-3283, 2012. https://doi.org/10.1021/om3001179
Tags: alkene, alkyne, Functional groups, intra-molecular search, search query
I noted a close C-H…π contact in ICUTAC (QA = C was excluded from the search), so here it is now.

An interesting structure XIRTUN (article DOI: 10.1021/ja001706w) is shown below, in which chloroform provides the “acidic” hydrogen. The angle H—centroid—H angle is 86°.
A 3D model of XEZHER is at dataDOI: 10.5517/CCYZ1SQ . The H…centroid distance is 2.16Å.