Following my conformational exploration of enols, here is one about a much more common molecule, a carboxylic acid.
The components of the search are shown as four queries below, which will be combined in various Boolean senses (DOI: 10.14469/hpc/2462).
- Query one defines the carboxylic acid, with 3-coordinate carbon specified at the carbonyl along with 1-coordinate for the carbonyl oxygen. Then the HO-C=O torsion (o° for the syn conformation shown on the left above and 180° for the anti-conformation shown on the right) and the length of the O-C bond as variables.
- Query two defines a contact as ≤ the sum of van der Waals radii between QA (=N,O,F,Cl) and the hydrogen of the carboxylic acid (pink).
- Query three defines a contact as ≤ the sum of van der Waals radii between QA-H (QA=N,O,F,Cl) and the oxygen of the acid (pink).
- Query four defines a temperature of <100K for the data collection temperature.
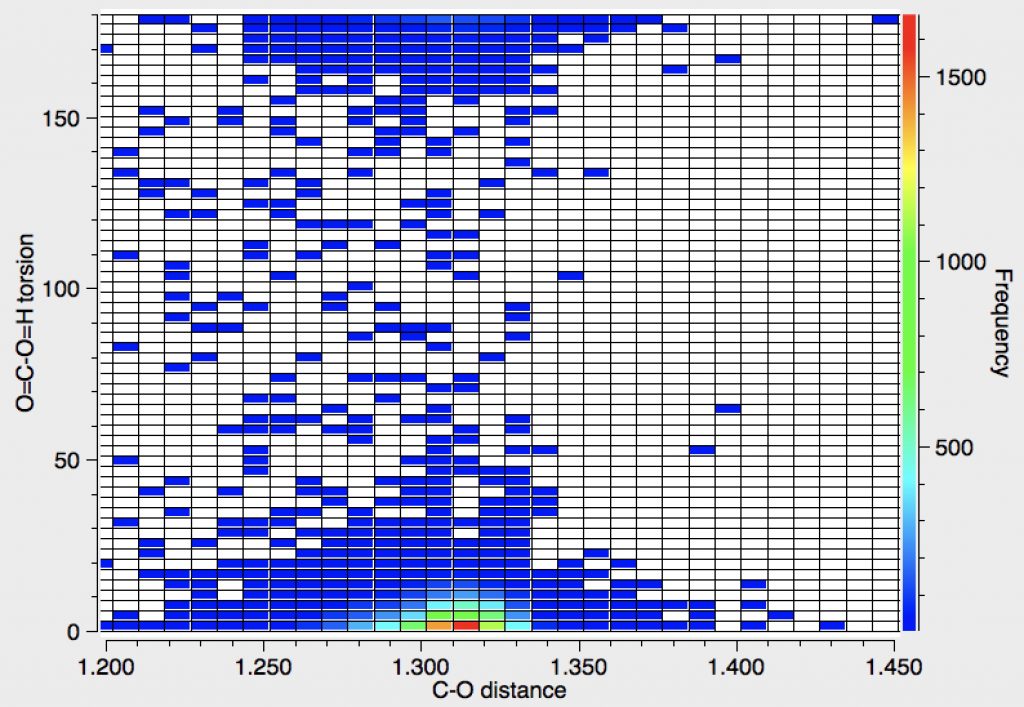
The first search uses just Query 1, with additional constraints of no errors, no disorder and R < 0.05.
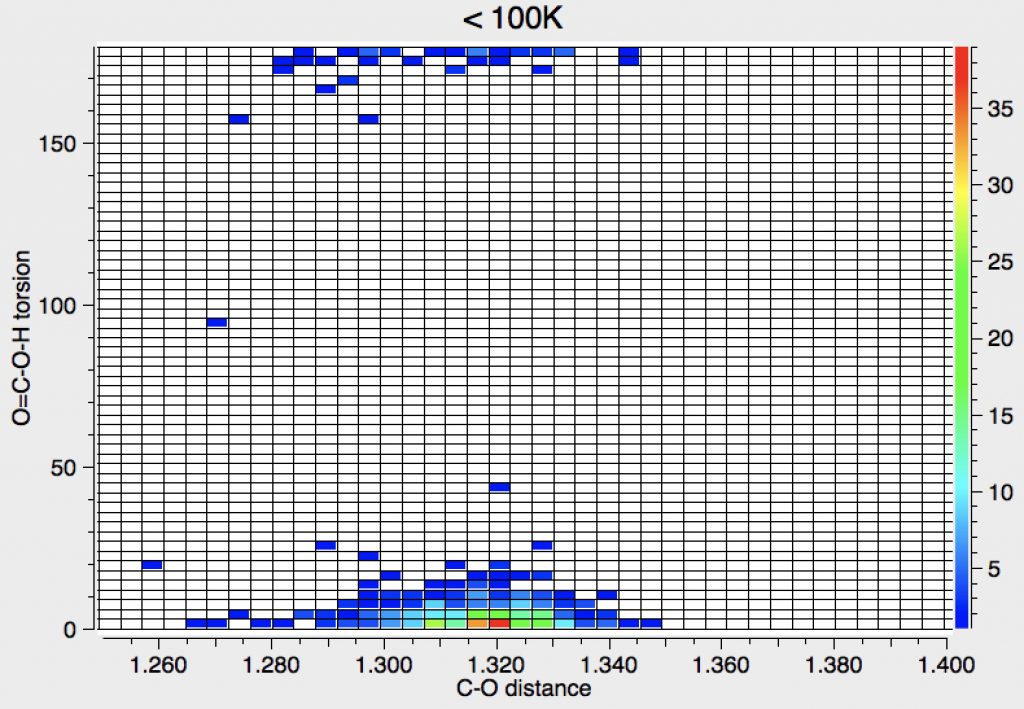
This can then be focused by combining Query 1 + Query 4, which shows a clear preference for the syn conformation.
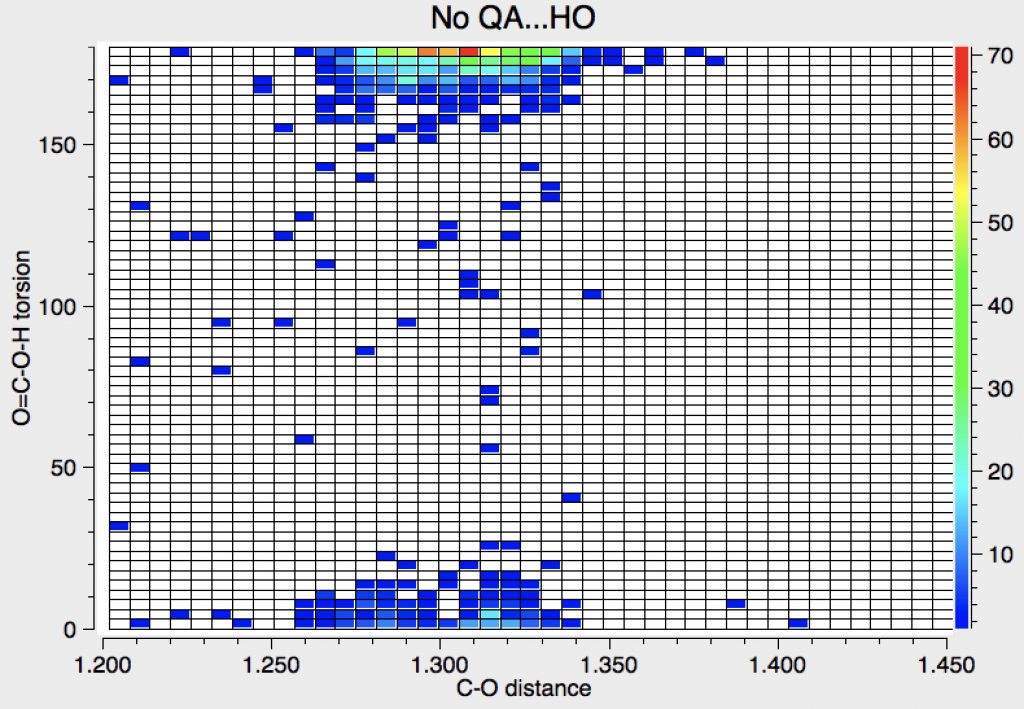
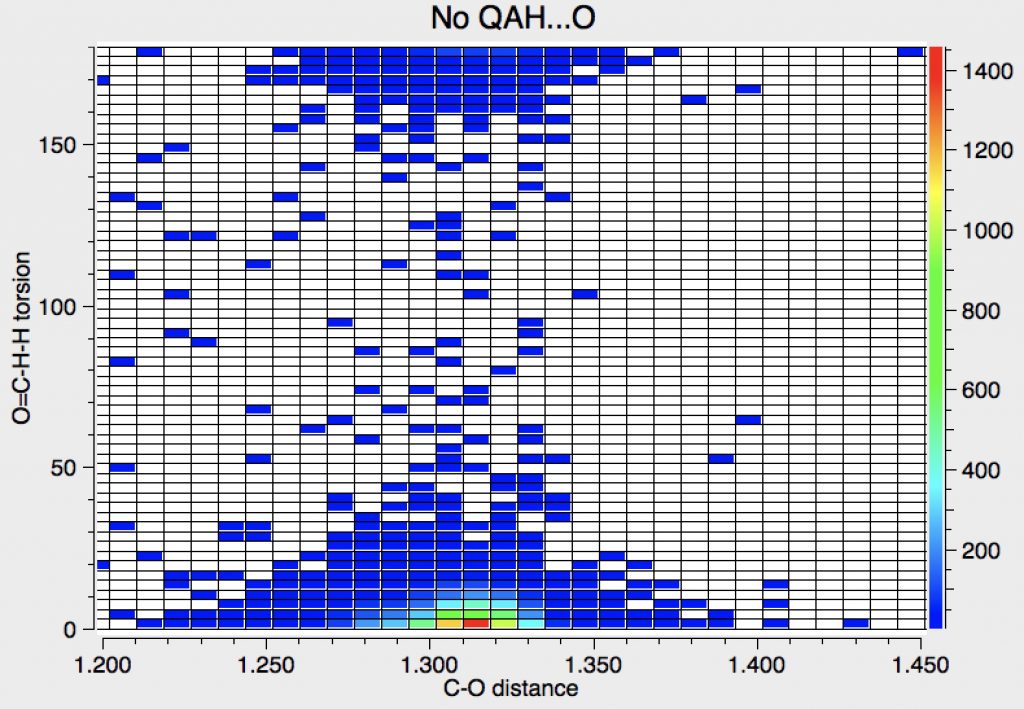
Next, Query 1 with NOT query 2, which restricts the search to carboxylic acids that do not have contacts to the hydrogen of the OH group. This excludes carboxylic acid dimers, as shown above. The predominant hot-spot now corresponds to the anti conformation.
Again this is narrowed using Query 4, which removes almost all the syn examples.
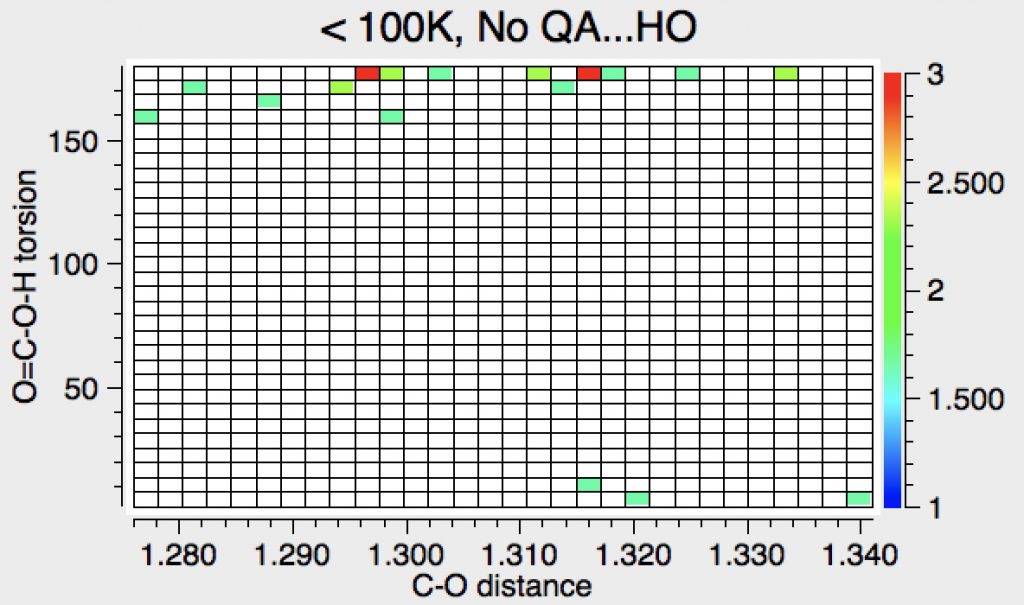
Now using Query 3 (as shown above), which restricts the search to examples where the oxygen of the HO group is itself not in contact with an acidic hydrogen. This allows carboxylic acid dimers. This now reveals the syn preference again.
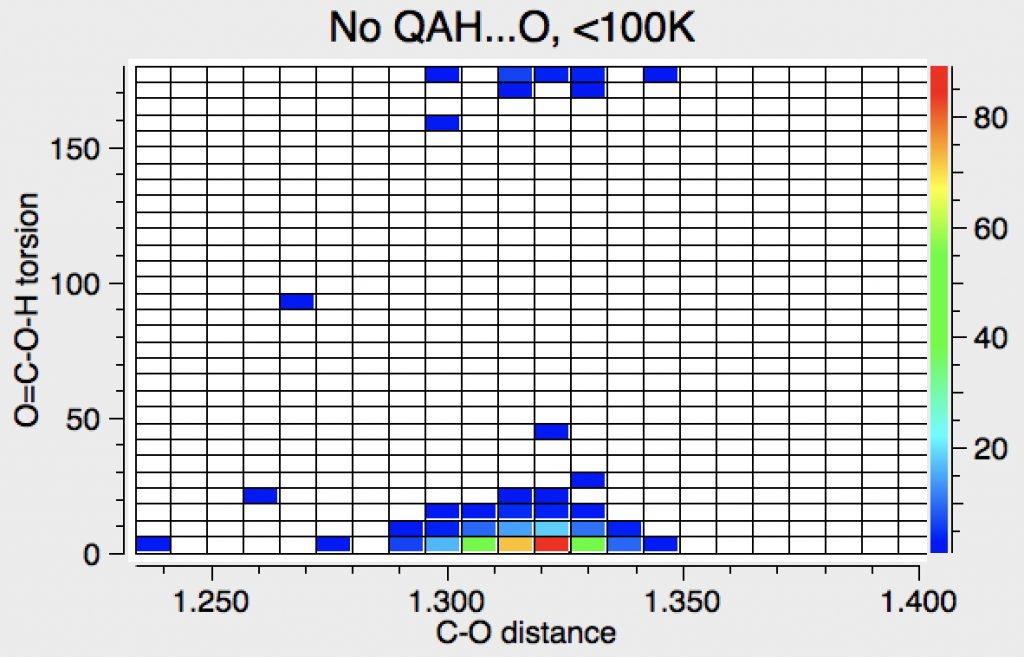
At <100K reinforces this effect.
Finally, Query 1 and NOT query 2 (no dimers) and NOT query 3, where a smaller preference for anti is seen.
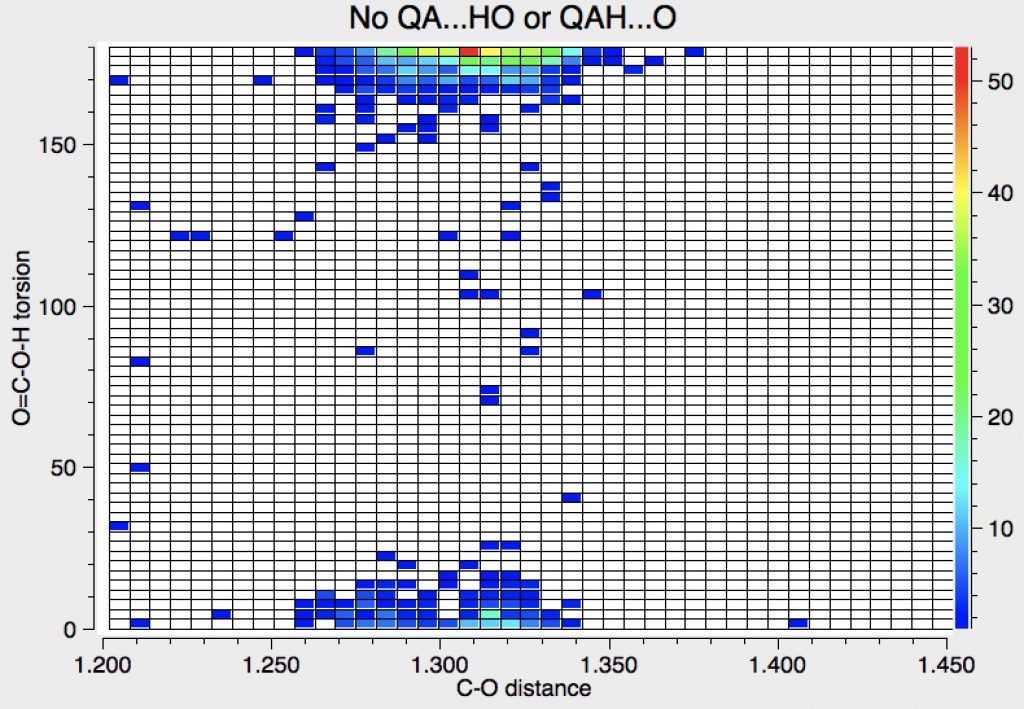
So it seems that an interesting difference emerges between enols and carboxylic acids in that when no hydrogen bonding to the HO group is allowed, an anti preference emerges. The electronic origins of this effect will be probed in a future post.
Tags: Acid, Alcohols, carboxylic acid, Chemistry, Enol, Functional groups, Organic chemistry, search uses