In my earlier post on the topic, I discussed how inverting the polarity of the C-X bond from X=O to X=Be could flip the stereochemical course of the electrocyclic pericyclic reaction of a divinyl system. An obvious question would be: what happens at the half way stage, ie X=CH2? Well, here is the answer.
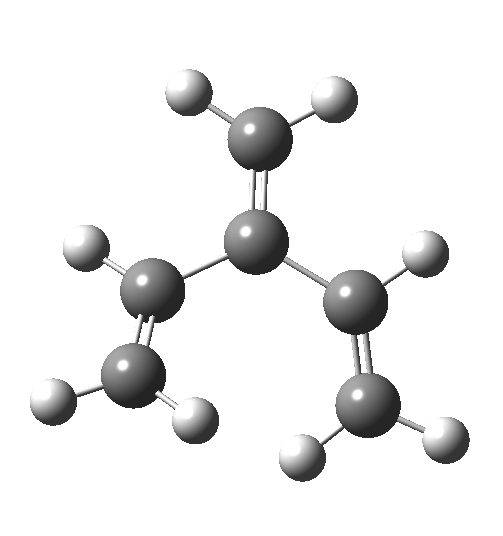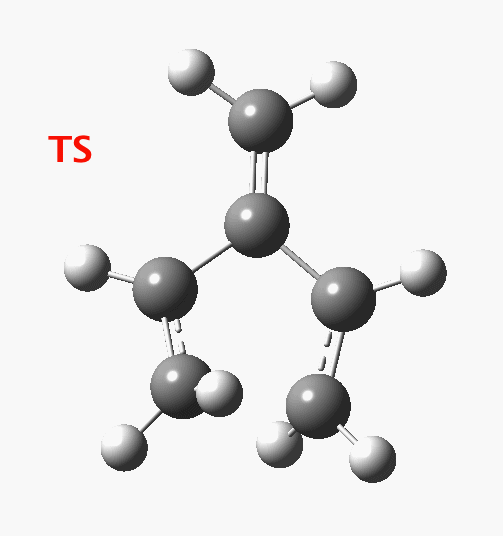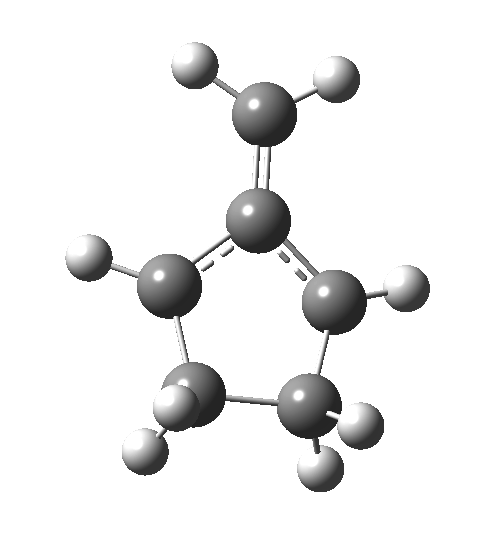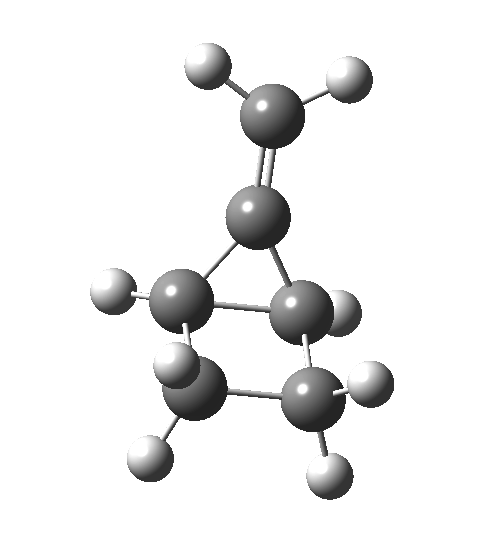 The reaction occurs in two stages (ωB97XD/6-311G(d,p)/SCRF=dichloromethane)[1] but overall is a concerted, albeit asynchronous, reaction. The initial stage is a conrotatory ring closure (as observed with X=O but opposite to X=Be), and reaching what we will call a HI (hidden intermediate). This HI clearly has zwitterionic character, and manifests its presence most obviously at IRC = -3.5 below.
The reaction occurs in two stages (ωB97XD/6-311G(d,p)/SCRF=dichloromethane)[1] but overall is a concerted, albeit asynchronous, reaction. The initial stage is a conrotatory ring closure (as observed with X=O but opposite to X=Be), and reaching what we will call a HI (hidden intermediate). This HI clearly has zwitterionic character, and manifests its presence most obviously at IRC = -3.5 below. The polarity of this HI is revealed by the dipole moment (6D) and molecular electrostatic potentials, below. The dipole vector goes from -ve to +ve, and the MEP clearly reveals the polarity below.
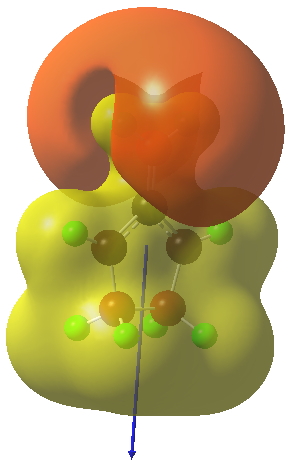 This ionic HI however is not stable, and in the second stage of the reaction collapses to the neutral bicyclic hydrocarbon shown below. Overall, it amounts to a 2+2 cycloaddition, but with a very unusual pathway in which one C-C bond is very much formed before the other (which is how the reaction escapes the clutches of the Woodward-Hoffmann forbidden-ness).
This ionic HI however is not stable, and in the second stage of the reaction collapses to the neutral bicyclic hydrocarbon shown below. Overall, it amounts to a 2+2 cycloaddition, but with a very unusual pathway in which one C-C bond is very much formed before the other (which is how the reaction escapes the clutches of the Woodward-Hoffmann forbidden-ness). Why is all this worth this follow-up? Well, one can now start to “design” the reaction. All three carbon atoms with formal charges can be stabilised or destabilised with appropriate substituents. It should not be too difficult to stabilise out the HI into just an I(intermediate), or indeed to remove it from the profile. Nice perhaps for a group of students, who can partition up the substituents amongst themselves and discover if they have the desired effect. And would any of this tinkering change the stereochemical outcome?
References
- H.S. Rzepa, "Gaussian Job Archive for C6H8", 2014. https://doi.org/10.6084/m9.figshare.1128205
Tags: Hawaii