I was lucky enough to attend the announcement made in 2012 of the discovery of the Higgs Boson. It consisted of a hour-long talk mostly about statistics, and how the particle physics community can only claim a discovery when their data has achieved a 5σ confidence level. This represents a 1 in 3.5 million probability of the result occurring by chance. I started thinking: how much chemistry is asserted at that level of confidence? Today, I read Steve Bachrach’s post on the structure of Citrinalin B and how “use of Goodman’s DP4 method indicates a 100% probability that the structure of citrinalin B is (the structure below)”. Wow, that is even higher than the physicists. Of course, 100% has been obtained by rounding 99.7 (3σ is 99.73%) or whatever (this is one number that should never be so rounded!). 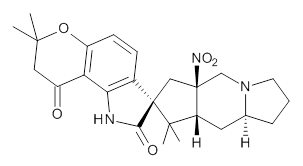 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
- I think the confidence level for assigning absolute configurations on the basis of ECD analogy with other compounds is the lowest of all the methods. Around 1σ or 68.3% (and this mostly from additional information such as the chemical transforms performed from starting materials of known absolute configuration).
- VCD is higher. If performed on the actual compound, I think it can be as high as 2-3σ or 95.5-99.7%. It is difficult to know how much of this certainty is lost by using only model compounds.
- Flack analysis (of anomalous X-ray)[5] is probably also at 2-3σ; I suggest however that a fair bit of uncertainly not included in the 2-3σ probably arises from analysing a tiny crystal (1 µg) arising from a solution perhaps 10,000 times larger in weight of sample.
- And of course combining the uncertainties from multiple experiments reduces it overall.
I am not casting any doubts on an assigned absolute configuration on which that of citrinalin B is based, as done in 2005. I have no grounds to think it is wrongly assigned. I am merely suggesting that in 2014, one should be able to achieve an even greater confidence level. And do what the physicists do, try to estimate the confidence level attained. I wonder how much chemistry would match the physicists 5σ-confidence level (99.99994%)?
References
- E.V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E.F. Pimenta, D.K. Romney, M.W. Lodewyk, D.E. Williams, R.J. Andersen, S.J. Miller, D.J. Tantillo, R.G.S. Berlinck, and R. Sarpong, "Total synthesis and isolation of citrinalin and cyclopiamine congeners", Nature, vol. 509, pp. 318-324, 2014. https://doi.org/10.1038/nature13273
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao, and J. Kobayashi, "Absolute Stereochemistry of Citrinadins A and B from Marine-Derived Fungus", The Journal of Organic Chemistry, vol. 70, pp. 9430-9435, 2005. https://doi.org/10.1021/jo051499o
- F. Cherblanc, Y. Lo, E. De Gussem, L. Alcazar‐Fuoli, E. Bignell, Y. He, N. Chapman‐Rothe, P. Bultinck, W.A. Herrebout, R. Brown, H.S. Rzepa, and M.J. Fuchter, "On the Determination of the Stereochemistry of Semisynthetic Natural Product Analogues using Chiroptical Spectroscopy: Desulfurization of Epidithiodioxopiperazine Fungal Metabolites", Chemistry – A European Journal, vol. 17, pp. 11868-11875, 2011. https://doi.org/10.1002/chem.201101129
- F.L. Cherblanc, Y. Lo, W.A. Herrebout, P. Bultinck, H.S. Rzepa, and M.J. Fuchter, "Mechanistic and Chiroptical Studies on the Desulfurization of Epidithiodioxopiperazines Reveal Universal Retention of Configuration at the Bridgehead Carbon Atoms", The Journal of Organic Chemistry, vol. 78, pp. 11646-11655, 2013. https://doi.org/10.1021/jo401316a
- H.D. Flack, and G. Bernardinelli, "The use of X‐ray crystallography to determine absolute configuration", Chirality, vol. 20, pp. 681-690, 2007. https://doi.org/10.1002/chir.20473
Tags: chemical, Reading, Steve Bachrach, X-ray
Yesterday (11 February, 2016), the announcement of the detection of gravitational waves was made (doi: 10.1103/PhysRevLett.116.061102 ). The significance was > 5.1σ.
It continues to be interesting that in effect no chemistry is ever announced with a siginficance level quoted. I suspect that the synthesis of a new molecule, and its spectroscopy and crystallographic characterisation however must surely be > 5σ?
I noted above data recorded using LIGO revealed a gravitational wave at > 5.1σ significance. I feel I should also note that the data on which this is based is published at doi: 10.7935/K5MW2F23. It is of course entirely appropriate that this open science project should manage its data openly and in (from what I am able to judge) an exemplarly manner. However! I cannot resist but point out that the metadata associated with the LIGO event is not quite so exemplary, see http://data.datacite.org/10.7935/K5MW2F23