Continuing my european visits, here are two photos from Bonn. First, a word about how the representation of benzene evolved, attributed to Kekulé.
Above is his first effort, made in 1865.
This one above is better, offered in 1866. But whilst what we now know as the double bond (C=C) is perhaps understandably kinked (and we now call these banana bonds, since nature tends to abhor kinks in electron density), so too are the single bonds!
So when it came to erecting a statue in his honour around 1890, the kinks were straightened out! The figure on the right (female) represents science (and purity). The two chaps on the left are workers representing industry. Note that they have still not quite gotten the lengths of the putative single and double bonds in proportion. By 1872 of course, Kekulé had proposed[1],[2] his oscillating model, the one that is taught to this day.
I feel I should add one modern interpretation to this concept. An oscillation implies a frequency. Kekulé could only know that this oscillation was fast on what might be called the “laboratory scale” (in other words, no-one had been able to isolate the individual isomers of substituted benzenes, which implies that the rate constant inter-converting them was probably faster than k = 10-3 s-1 or a few minutes half-life).‡ We now know that this oscillation is ~1014 s-1, the timescale of a molecular vibration! By the way, transition state theory tells us that Ln(k/T) = 23.76 – ΔG‡/RT where k is the unimolecular rate constant, ΔG‡ the free energy barrier, T the temperature and R the gas constant. Setting ΔG‡ to zero gives us a rate constant of ~1014 s-1 (the barrier must be zero, or very close to it).
Here is the statue atop the plinth above. Apparently it is honoured by the students with robes and other accoutrements on special occasions.
‡Another famous timescale inference was by Beckmann in 1889[3] when he deduced the existence of a transient unseen intermediate in the (what he thought was) racemisation of menthone. That intermediate of course was the enol.
References
- A. Kekulé, "Ueber einige Condensationsproducte des Aldehyds", Justus Liebigs Annalen der Chemie, vol. 162, pp. 77-124, 1872. https://doi.org/10.1002/jlac.18721620110
- A. Kekulé, "Ueber einige Condensationsproducte des Aldehyds", Justus Liebigs Annalen der Chemie, vol. 162, pp. 309-320, 1872. https://doi.org/10.1002/jlac.18721620211
- E. Beckmann, "Untersuchungen in der Campherreihe", Justus Liebigs Annalen der Chemie, vol. 250, pp. 322-375, 1889. https://doi.org/10.1002/jlac.18892500306
Tags: /RT, free energy barrier, gas constant
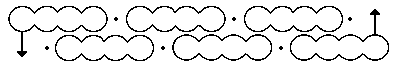


I have seen pictures of a mechanical model of the 1865 benzene depiction, also comprised of planar tetracoordinate (rather than tetrahedral) carbons.
Henry, I have read the Kekulé papers you cite, looking for “oscillation,” but never found it. Instead, he discussed “vibration” from a symmetrical (we would now call it “D6h”) structure. I concluded that others misinterpreted the idea Kekule was trying to convey (before its time) as “oscillation.” I’ve never located the reference where this was first done and by whom? Could you or your readers tell me?
I don’t think that Kekulé ever proposed the “oscillating model” you and others ascribe to hin. But who did so first? Kekulé did propose a “vibrating” model instead.
What was the first literature depiction of two D3h benzenes with two “equilibrium arrows” between them? It wasn’t by Kekulé.
I am interested in the origins of the understanding of molecular “vibrations”. Loschmidt apparently in 1861 discussed the motion of atoms about an equilibrium position, but the interaction between molecules and their excitation by light in the IR frequency range was not fully quantified until about 1905 when the first IR frequencies for typical bonds were reported by Coblentz (10.1103/PhysRevSeriesI.20.273). So it is not obvious that Kekule in 1966 or 1872 would have fully appreciated what the time scale of a vibration was. Nor would he have had any evidence of that timescale, or indeed been able to distinguish between benzene as a single minimum and benzene as a double minimum with a low enough barrier to prevent isolation of individual valence bond forms (that barrier could have been ~15 kcal/mol and would have accounted for the failure to separate valence bond isomers of substituted benzenes). I am not even sure that the concept of potential energy surfaces, and hence the idea of single or double minima was even articulated.
I think we are trying to overload the semantic meaning of “vibration” or “oscillation” as it might have been used in 1872. Neither would have had any frequency associated with it, and so they would have been interchangeable in the absence of further information.
It would be good to find the first use of “molecular vibration” in the literature that has a clear frequency range associated with the term. By how much does it predate Coblentz?
I have also tracked down two further, and dated, representations. The first from 1867 is a photograph of a physical model. The perspective seems to show tetrahedral carbon (the bottom double bond is obscured by perspective). Whilst it is very unlikely that tetrahedral carbon was really implied, the model is clearly not flat. It would be good to actually look at the physical model to verify.
The second appears to be dated 1872. I do not know if the original comes from local records, or from a published article. It is very much the modern form. But no relationship between the two (oscillation, vibration or other) accompanies the diagram.
Both these diagrams come from a brochure prepared for the Historische Statten der Chemie in 2014.
I would add a rider to the above. Whilst we now are absolutely certain that benzene is what is called a single minimum, and that the two forms above represent the limits of a vibration (indeed called the Kekulé mode), the clarity rapidly deteriorates for larger analogues of benzene. Indeed Paul Schleyer has written on this very problem (DOI: c5fdv9) for the molecule [18]-annulene. Is the Kekulé mode for this molecule a vibration? Or does it represent a transition state between two valence bond forms? Would one use the modern meaning of the term vibration for the first and (a much much slower) oscillation for the second? Nowadays the semantic interpretation might be that an oscillation is a slow process across a barrier connecting two minima and a vibration a very much faster process across a single minimum. But could that have been understood in 1872?