Paul Schleyer sent me an email about a pattern he had spotted, between my post on F3SSF and some work he and Michael Mauksch had done 13 years ago with the intriguing title “Demonstration of Chiral Enantiomerization in a Four-Atom Molecule“.[1] Let me explain the connection, but also to follow-up further on what I discovered in that post and how a new connection evolved.
The prologue (or prequel). Reaction 2 is the path for decomposing the dimer of SF2 (X=F) to two monomers. In the previous post I (eventually) found the transition state for this process, with a relatively low energy barrier. As a mechanistic type, it is known as a reductive elimination (the reverse would be a oxidative addition) since the S atom on the left is reduced from a formal oxidation state of S(IV) to S(II) (or vice versa). Analogues of this reaction are 1 and 3. But before I managed to locate the transition state for reaction 2, I accidentally found the transition state for reaction 4. This retains the S-S bond (at the transition state, this bond is actually shorter than in reactant/product), and is what might be called a two-electron pericyclic redox reaction, since the S on the left is reduced to S(II) and the S on the right is oxidised to S(IV). I have not yet found whether this actually represents a new mechanistic type or not; it does not appear to have a name (should it be called periredox? Or redoxocyclic?). The lesson to be learnt here is that nature normally indulges in the (more or less) lowest energy route to a given target, but quantum chemists have the advantage that they can discover “chemistry in the clouds”; patterns of behaviour requiring too much energy to be seen in the real world and hence permanently hidden from us. But that does not mean we cannot learn chemistry from them.
Thus isomeric reaction 4 is very much higher in energy than 2. But it is what triggered Paul’s memory. Reaction 5 is related both to 4 in that it involves a [1,2] hydrogen shift of X (retaining the S-S bond) followed by a second [1,2] shift of Y. It is also related to 2 since it involves in effect an oxidative addition (by a lone pair) to an S-X bond to generate S(IV), followed by a reductive elimination back to S(II) to regenerate the enantiomer of the reactant (it is thus a two-step redox reaction). Thus if X and Y are different in 5, then all three of the species shown above are themselves chiral, and hence the reaction is indeed a “Demonstration of Chiral Enantiomerization in a Four-Atom Molecule”. The point here is that enantiomerisations do not necessarily have to proceed through an achiral transition state, but that the entire enantiomerisation pathway can be continuously chiral.
That was the intro! Now follows my calculated intrinsic reaction coordinate (ωB97XD/6-311G(d,p) for reaction 5.[2] My first attempt at the transition state was to use 2 as a template (rather than 4, which was far higher in energy). Well, talk about unexpected! The migration of X=Cl is 16.7 kcal/mol lower than X=F. No problem there. Next, the IRC for X=F. The overall process certainly enantiomerises the two chiral gauche conformations, but without transposing X and Y, and not involving an intermediate S(IV) species as shown in reaction 5 (i.e. it goes directly, via reaction 6).
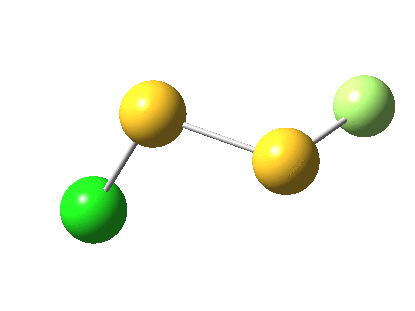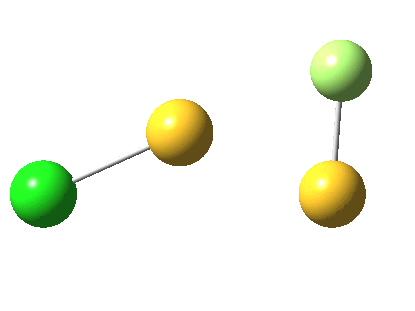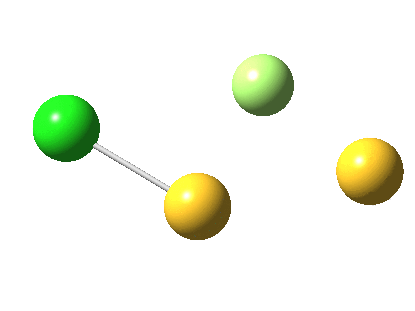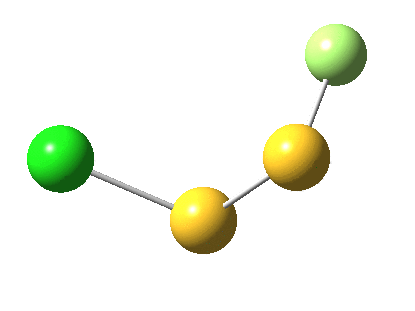
But look at that energy! Way too high (above the clouds in fact). And although the start and end species are identical (apart from being enantiomers) the energy profile is far from being symmetrical.
As for the gradient norms, where to begin? The TS as always is at IRC =0.0 But in between it and the start and end points one can see no less than THREE “hidden intermediates“. Two of them are in fact exactly cis (IRC=3.5) and trans (IRC = 5.0) planar forms of F-S-S-Cl. At these points, the pathway is clearly achiral! The third (IRC = 1.0) is a fascinating species in which the S-S bond is largely broken and it is bridged by an F. So this pathway involves S-S cleavage, just like 2. It is entirely serendipitous; no-one in their right mind would actually set out to find it!
Well, since 2 as a template led to the above, what happens when 4 is used? For F migrating[3] a barrier 11.6 kcal/mol higher is found than for Cl migrating[4], similar to that previously reported.[1]
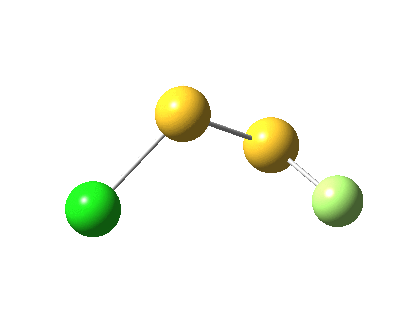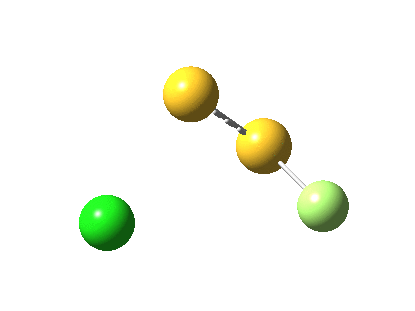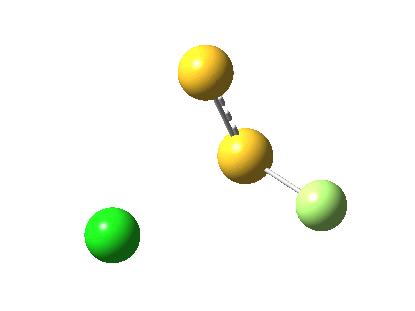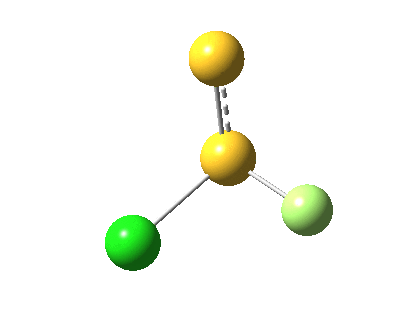
The energy and gradient norm profiles, in comparison to the previous, are uneventful.[5] The S-S bond stays intact throughout, and it is shorter at the transition state (1.846Å) than at the start (1.950Å) or the end (1.874Å). This reaction has got its feet on the ground, rather than its head in the clouds!
I am reminded of stories our crystallographer here tells. Students bring him synthesized molecules for their structures to be determined, and quite frequently it’s not at all the compound that was desired. For not a few highly focused students, the compound is quickly forgotten, even though it may have turned out to be very unusual. Likely it will not be deposited into a repository. And how many compounds that might otherwise have been the catalyst for new and unusual discoveries are thus lost? So never throw away an unexpected result (yes, even a calculation). There is probably something you could learn from it!
References
- H.S. Rzepa, "Gaussian Job Archive for ClFS2", 2013. https://doi.org/10.6084/m9.figshare.801866
- H.S. Rzepa, "Gaussian Job Archive for ClFS2", 2013. https://doi.org/10.6084/m9.figshare.803096
- H.S. Rzepa, "Gaussian Job Archive for ClFS2", 2013. https://doi.org/10.6084/m9.figshare.802822
- H.S. Rzepa, "Gaussian Job Archive for ClFS2", 2013. https://doi.org/10.6084/m9.figshare.802821
Tags: energy, energy profile, head, low energy barrier, lowest energy route, Michael Mauksch, Paul Schleyer, reactant/product
A follow up to my comment that X-S-S-Y was chiral (if X=Y, it would have C2 disymetric symmetry because it adopted the gauche conformation. Here is a CCDD search, using the following definition (QA = any from groups 15-17).
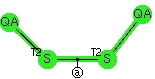
The results are shown below. Pretty clear cut!
A similar distribution is seen when the two central atoms are any combination of Se and Te.
[…] Chemistry with a twist « Patterns of behaviour: serendipity in action for enantiomerisation of F-S-S-Cl […]
Henry, my hat is off to you. Valuable information and excellent clarity you got here! I find your information very helpful. It may help me about related matter. Thanks for sharing…