Here is another example gleaned from that Woodward essay of 1967 (Chem. Soc. Special Publications (Aromaticity), 1967, 21, 217-249), where all might not be what it seems.
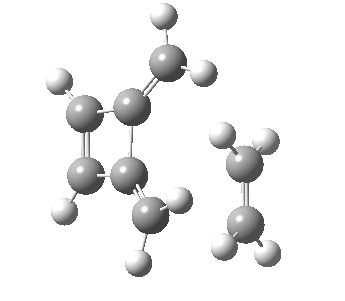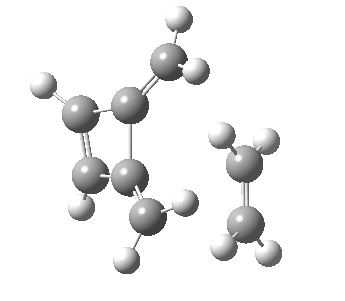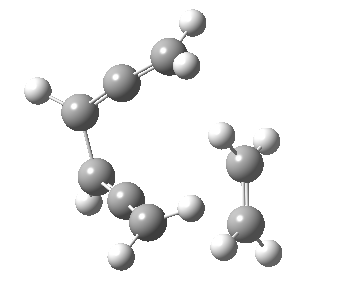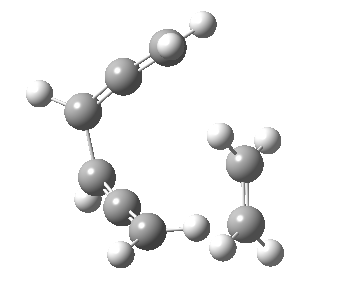
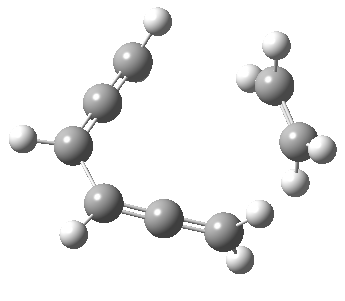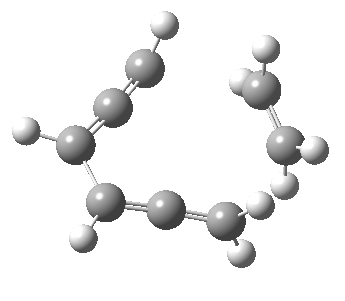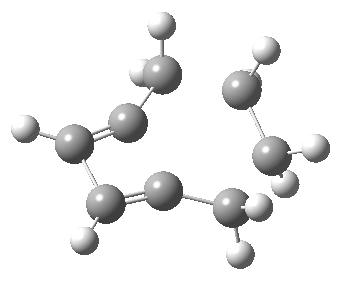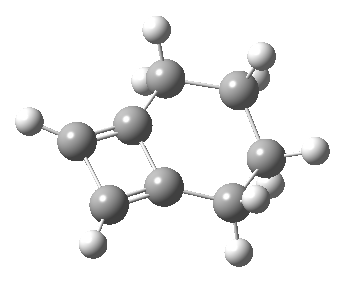
Woodward notes that the reaction between the (highly reactive) 1 does not occur. This is attributed to it being a disallowed π6 + π2 cycloaddition (blue + magenta arrows) rather than an allowed π4 + π2 cycloaddition (red + magenta arrows). So what does quantum mechanics say? Well, a disallowed reaction can be broken down into several stages, each involving fewer electrons, and this is what happens. The first of these stages becomes instead an electrocyclic ring opening (green arrows) in which one σ-bond from the cyclobutene is “borrowed” to form a bis-allene intermediate, before being returned to the original bond in the second stage.
| Electrocyclic ring opening[1] | |
|---|---|
 |
|
| 2+2+2 cycloaddition[2] | |
 |
|
The first transition state for ring opening proceeds in the appropriate Woodward-Hoffmann conrotatory mode, and has a free energy barrier of ~ 45 kcal/mol. This is still 46.1 kcal/mol lower than the very unfavourable second step, which involves a 2+2+2 cycloaddition. Both are formally symmetry-allowed reactions, they just have very high barriers to reaction which accounts for the non-occurance experimentally. Of course, one interpretation of the WH rules is that any pericyclic with a high barrier could be regarded as forbidden, but in this case not on the grounds of symmetry.
References
- H.S. Rzepa, "Gaussian Job Archive for C8H10", 2013. https://doi.org/10.6084/m9.figshare.705831
- H.S. Rzepa, "Gaussian Job Archive for C8H10", 2013. https://doi.org/10.6084/m9.figshare.705830
Tags: free energy barrier, Historical, Reaction Mechanism, Tutorial material