Posts Tagged ‘Stereochemistry’
Friday, July 1st, 2016
The anomeric effect occurs at 4-coordinate (sp3) carbon centres carrying two oxygen substituents and involves an alignment of a lone electron pair on one oxygen with the adjacent C-O σ*-bond of the other oxygen. Here I explore whether other centres can exhibit the phenomenon. I start with 4-coordinate boron, using the crystal structure search definition below (along with R < 0.1, no disorder, no errors).[cite]10.14469/hpc/696[/cite]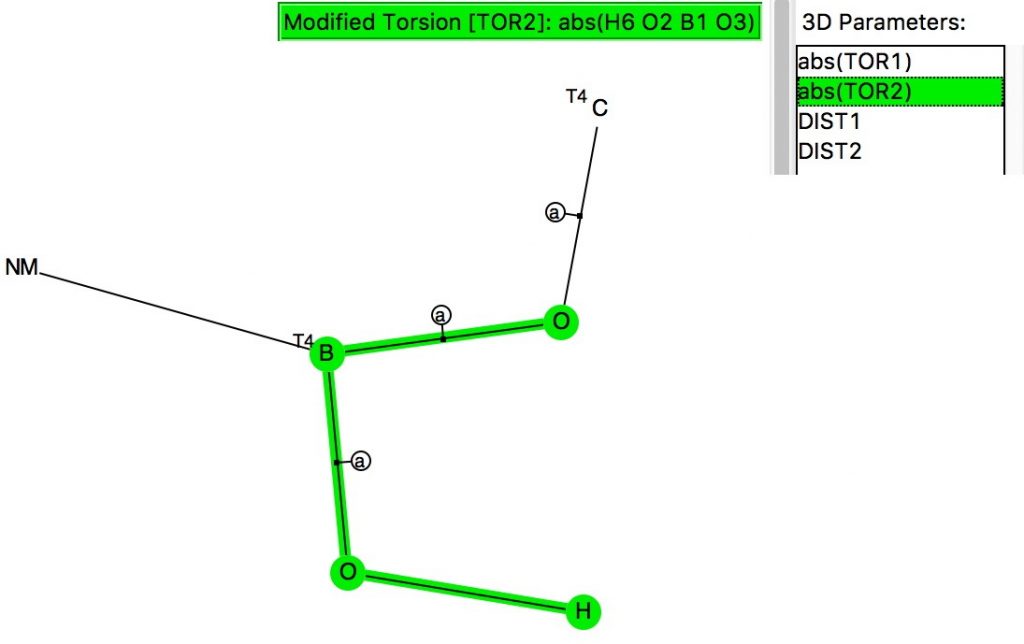
(more…)
Tags:Acetals, Alkane stereochemistry, Anomer, Anomeric effect, Bond length, Boron, Carbohydrate, Carbohydrate chemistry, Carbohydrates, crystal structure search definition, Ester, Physical organic chemistry, Stereochemistry
Posted in crystal_structure_mining | No Comments »
Monday, May 30th, 2016
This is a follow-up to one aspect of the previous two posts dealing with nucleophilic substitution reactions at silicon. Here I look at the geometries of 5-coordinate compounds containing as a central atom 4A = Si, Ge, Sn, Pb and of the specific formula C34AO2 with a trigonal bipyramidal geometry. This search arose because of a casual comment I made in the earlier post regarding possible cooperative effects between the two axial ligands (the ones with an angle of ~180 degrees subtended at silicon). Perhaps the geometries might expand upon this comment?
(more…)
Tags:Anomer, Anomeric effect, Carbohydrate chemistry, Carbohydrates, Ligand, Molecular geometry, Physical organic chemistry, Stereochemistry, Stereoelectronic effect, Trigonal bipyramidal molecular geometry
Posted in Chemical IT, crystal_structure_mining | 3 Comments »
Wednesday, May 25th, 2016
The substitution of a nucleofuge (a good leaving group) by a nucleophile at a carbon centre occurs with inversion of configuration at the carbon, the mechanism being known by the term SN2 (a story I have also told in this post). Such displacement at silicon famously proceeds by a quite different mechanism, which I here quantify with some calculations.
(more…)
Tags:Berry mechanism, Elimination reaction, energy, energy barrier, energy profile, free energy, Leaving group, lower energy orientation, Molecular geometry, Organic reactions, overall free energy, Pseudorotation, search query, SN2 reaction, Stereochemistry, Trigonal bipyramidal molecular geometry
Posted in reaction mechanism | No Comments »
Saturday, January 16th, 2016
The post on applying VSEPR ("valence shell electron pair repulsion") theory to the geometry of ClF3 has proved perennially popular. So here is a follow-up on another little molecue, F3SN. As the name implies, it is often represented with an S≡N bond. Here I take a look at the conventional analysis.
(more…)
Tags:Chemical bond, chemical bonding, Electron, Lone pair, Molecular geometry, Octet rule, Quantum chemistry, Stereochemistry, Tetrahedral molecular geometry, Theoretical chemistry, Valence, VSEPR theory
Posted in Hypervalency | 110 Comments »
Thursday, August 27th, 2015
The anomeric effect is best known in sugars, occuring in sub-structures such as RO-C-OR. Its origins relate to how the lone pairs on each oxygen atom align with the adjacent C-O bonds. When the alignment is 180°, one oxygen lone pair can donate into the C-O σ* empty orbital and a stabilisation occurs. Here I explore whether crystal structures reflect this effect.
(more…)
Tags:Alkane stereochemistry, Anomeric effect, Carbohydrate chemistry, Carbohydrates, Carbon–oxygen bond, Chemical bond, Ether, Lone pair, Physical organic chemistry, Quantum chemistry, Stereochemistry, Technology/Internet
Posted in Chemical IT, crystal_structure_mining | No Comments »
