In the last post, IH7 was examined to see if it might exhibit true hypervalency. The iodine, despite its high coordination, turned out not to be hypervalent, with its (s/p) valence shell not exceeding eight electrons (and its d-shell still with 10, and the 6s/6p shells largely unoccupied). Instead, the 14 valence electrons (7 from H, 7 from iodine) fled to the H…H regions. Well, perhaps H is special in its ability to absorb electrons into the H…H regions. So how about I(CN)7? (the species has not hitherto been reported in the literature according to CAS). The cyano group is often described as a pseudohalide, but the advantage of its use here is that it is about the same electronegativity as I itself, and hence the I-C bond is more likely to be covalent (than for example an I-F bond). As noted in the earlier blog, if the potentially hypervalent atom is very ionic, it can be difficult to know whether the electrons are truly associated with that atom, or whether they are in fact in lone pairs associated with the other electronegative atom (e.g. F). It is also important to avoid large substituents, otherwise steric interactions will cause problems around the equator.
Posts Tagged ‘pence’
Hypervalency: I(CN)7 is not hypervalent!
Sunday, October 17th, 2010Hypervalency: Is it real?
Saturday, October 16th, 2010The Wikipedia page on hypervalent compounds reveals that the concept is almost as old as that of normally valent compounds. The definition there, is “a molecule that contains one or more main group elements formally bearing more than eight electrons in their valence shells” (although it could equally apply to e.g. transition elements that would contain e.g. more than 18 electrons in their valence shell). The most extreme example would perhaps be of iodine (or perhaps xenon). The normal valency of iodine is one (to formally complete the octet in the valence shell) but of course compounds such as IF7 imply the valency might reach 7 (and by implication that the octet of electrons expands to 14). So what of IF7? Well, there is a problem due to the high electronegativity of the fluorine. One could argue that the bonds in this molecule are ionic, and hence that the valence electrons really reside in lone pairs on the F. Thus the apparently hypervalent PF5 could be written PF4+…F–, in which case the P is not really hypervalent after all. We need a compound with un-arguably covalent bonds. Well, what about IH7? One might probably still argue about ionicity (for example H+…IH6–) but that puts electrons on I and not H, and hence does not change any hypervalency on the iodine. Surely, if hypervalency is a real phenomenon, it should manifest in IH7?
Quintuple bonds
Tuesday, February 16th, 2010Climbers scale Mt. Everest, because its there, and chemists have their own version of this. Ever since G. N. Lewis introduced the concept of the electron-pair bond in 1916, the idea of a bond as having a formal bond-order has been seen as a useful way of thinking about molecules. The initial menagerie of single, double and triple formal bond orders (with a few half sizes) was extended in the 1960s to four, and in 2005 to five. Since then, something of a race has developed to produce the compound with the shortest quintuple bond. One of the candidates for this honour is shown below (2008, DOI: 10.1002/anie.200803859) which is a crystalline species (a few diatomics which exist in the gas phase are also candidates; for other reviews of the topic see 10.1038/nchem.359, 10.1021/ja905035f and 10.1246/cl.2009.1122).
Clar islands in a π Cloud
Wednesday, December 9th, 2009Clar islands are found not so much in an ocean, but in a type of molecule known as polycyclic aromatic hydrocarbons (PAH). One member of this class, graphene, is attracting a lot of attention recently as a potential material for use in computer chips. Clar coined the term in 1972 to explain the properties of PAHs, and the background is covered in a recent article by Fowler and co-workers (DOI: 10.1039/b604769f). The concept is illustrated by the following hydrocarbon:
Towards the ultimate bond!
Monday, August 24th, 2009
The 100th anniversary of G. N. Lewis’ famous electron pair theory of bonding is rapidly approaching in 2016 (DOI: 10.1021/ja02261a002). He set out a theory of bond types ranging from 1-6 electrons. The strongest bond recognized by this theory was the 6-electron triple bond, a good example of which occurs in dinitrogen, N2. In terms of valence electrons, nitrogen has an atomic configuration of 2s2, 2p3. Each atom has five electrons in total, some or all of which in principle could be used for forming bonds. An exploration of this motif across the entire periodic table is presented in part one of this blog.
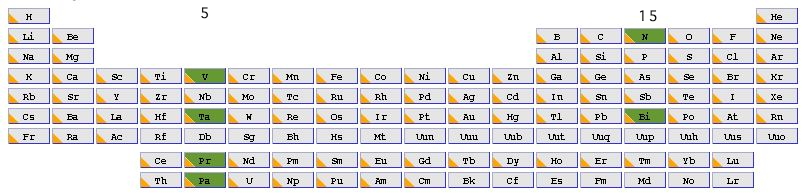
Nitrogen is in the main group 15, and the element at the bottom of this group is Bismuth (also with the same atomic configuration). We can then move to the corresponding column of the transition series, this time occupying group 5. The first examplar in this set, Vanadium has an atomic configuration of 3d3, 4s2, again five valence electrons, but now utilizing the d- rather than the p-shell of valence atomic orbitals (AOs). The final forage across the period table would land us with Pr and Pa, which occupy the lanthanide and actinide series respectively, and which have atomic configurations of 4f3, 6s2 and 5f2, 6d1 and 7s2 respectively. You can now see the theme developing; how does the bonding develop between two atoms that between them have ten valence electrons occupying molecular orbitals constructed from s, and then either p, d or f atomic orbitals. The next in that series, g atomic orbitals, are thought unlikely to have any chemical significance in the presently known periodic table.
Molecular toys: Tetrahedral cavities
Saturday, July 4th, 2009
An earlier post described how a (spherical) halide anion fitted snugly into a cavity generated by the simple molecule propanone, itself assembled by sodium cations coordinating to the oxygen. A recent elaboration of this theme, reminiscent of the children’s toys where objects have to be fitted into the only cavity that matches their shape, Nitschke and co-workers report the creation of a molecule with a tetrahedral rather than a spherical cavity (DOI: 10.1126/science.1175313 ), into which another but much smaller tetrahedral molecule is fitted. The small molecule is P4, in which each of the three valencies of the P atom is directed to a corner of the tetrahedron. The large molecule comprises four Fe atoms. These are each octahedrally coordinated with six ligand sites, three of which mimic the P atoms in also being directed towards the remaining three vertices of a tetrahedron.