Posts Tagged ‘Organic chemistry’
Friday, March 31st, 2017
Nowadays, data supporting most publications relating to the synthesis of organic compounds is more likely than not to be found in associated “supporting information” rather than the (often page limited) article itself. For example, this article[1] has an SI which is paginated at 907; almost a mini-database in its own right!† Here I ponder whether such dissemination of data is FAIR (Findable, accessible, interoperable and re-usable).[2]
(more…)
References
-
J.M. Lopchuk, K. Fjelbye, Y. Kawamata, L.R. Malins, C. Pan, R. Gianatassio, J. Wang, L. Prieto, J. Bradow, T.A. Brandt, M.R. Collins, J. Elleraas, J. Ewanicki, W. Farrell, O.O. Fadeyi, G.M. Gallego, J.J. Mousseau, R. Oliver, N.W. Sach, J.K. Smith, J.E. Spangler, H. Zhu, J. Zhu, and P.S. Baran, "Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity", Journal of the American Chemical Society, vol. 139, pp. 3209-3226, 2017. http://dx.doi.org/10.1021/jacs.6b13229
-
M.D. Wilkinson, M. Dumontier, I.J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J. Boiten, L.B. da Silva Santos, P.E. Bourne, J. Bouwman, A.J. Brookes, T. Clark, M. Crosas, I. Dillo, O. Dumon, S. Edmunds, C.T. Evelo, R. Finkers, A. Gonzalez-Beltran, A.J. Gray, P. Groth, C. Goble, J.S. Grethe, J. Heringa, P.A. ’t Hoen, R. Hooft, T. Kuhn, R. Kok, J. Kok, S.J. Lusher, M.E. Martone, A. Mons, A.L. Packer, B. Persson, P. Rocca-Serra, M. Roos, R. van Schaik, S. Sansone, E. Schultes, T. Sengstag, T. Slater, G. Strawn, M.A. Swertz, M. Thompson, J. van der Lei, E. van Mulligen, J. Velterop, A. Waagmeester, P. Wittenburg, K. Wolstencroft, J. Zhao, and B. Mons, "The FAIR Guiding Principles for scientific data management and stewardship", Scientific Data, vol. 3, 2016. http://dx.doi.org/10.1038/sdata.2016.18
Tags:Carbon, chemical databases, chemical graveyard, Chemical IT, chemical spectra, Chemistry, digital signature, Nature, Organic, Organic chemistry, Organic compound, Organic food, search engines, Technology/Internet
Posted in Uncategorised | 3 Comments »
Thursday, December 1st, 2016
Following on from a search for long C-C bonds, here is the same repeated for C=C double bonds.
(more…)
Tags:Chemical bond, chemical bonding, Chemical nomenclature, Chemistry, Conjugated system, double bond, energy, Interesting chemistry, Nature, Nonmetal, Organic chemistry, Physical organic chemistry, search query, Substituent
Posted in crystal_structure_mining | 2 Comments »
Monday, October 31st, 2016
Is asking a question such as “what is the smallest angle subtended at a chain of three connected 4-coordinate carbon atoms” just seeking another chemical record, or could it unearth interesting chemistry?
(more…)
Tags:animation, Bicyclic molecule, chemical record, Chemistry, City: Cambridge, Cycloalkane, Cyclopropanes, Java, Molecular geometry, Organic chemistry, potential energy surface, Safari, Web browser, X-ray
Posted in crystal_structure_mining, reaction mechanism | 7 Comments »
Wednesday, September 28th, 2016
The story so far. Imines react with a peracid to form either a nitrone (σ-nucleophile) or an oxaziridine (π-nucleophile).[1] The balance between the two is on an experimental knife-edge, being strongly influenced by substituents on the imine. Modelling these reactions using the “normal” mechanism for peracid oxidation did not reproduce this knife-edge, with ΔΔG (π-σ) 16.2 kcal/mol being rather too far from a fine balance.
(more…)
References
-
D.R. Boyd, P.B. Coulter, N.D. Sharma, W. Jennings, and V.E. Wilson, "Normal, abnormal and pseudo-abnormal reaction pathways for the imine-peroxyacid reaction", Tetrahedron Letters, vol. 26, pp. 1673-1676, 1985. http://dx.doi.org/10.1016/S0040-4039(00)98582-4
Tags:addition product, free-energy pathway, Functional groups, Imine, Nitrone, Nucleophile, Organic chemistry, Oxaziridine
Posted in reaction mechanism | No Comments »
Thursday, September 22nd, 2016
Compounds with O-O bonds often have weird properties. For example, artemisinin, which has some fascinating stereoelectronics. Here is another such, recently in the news and known as HMTD (hexamethylene triperoxide diamine). The crystal structure was reported some time ago[1] and the article included an inspection of the computed wavefunction. However this did not look at the potential stereoelectronics in this species, which I now address here.
(more…)
References
-
A. Wierzbicki, E.A. Salter, E.A. Cioffi, and E.D. Stevens, "Density Functional Theory and X-ray Investigations of P- and M-Hexamethylene Triperoxide Diamine and Its Dialdehyde Derivative", The Journal of Physical Chemistry A, vol. 105, pp. 8763-8768, 2001. http://dx.doi.org/10.1021/jp0123841
Tags:Amines, Artemisinin, Chemistry, Functional groups, Hexamethylene triperoxide diamine, Interesting chemistry, Organic chemistry, Organic peroxides, Peroxide, perturbation energy interaction, Stereoelectronics
Posted in Uncategorised | 1 Comment »
Tuesday, February 9th, 2016
The phenomenon of bond stretch isomerism, two isomers of a compound differing predominantly in just one bond length, is one of those chemical concepts that wax and occasionally wane.[1] Here I explore such isomerism for the elements Ge, Sn and Pb.
(more…)
References
-
J.A. Labinger, "Bond-stretch isomerism: a case study of a quiet controversy", Comptes Rendus. Chimie, vol. 5, pp. 235-244, 2002. http://dx.doi.org/10.1016/S1631-0748(02)01380-2
Tags:a Jahn-Teller, Bond length, chemical concepts, Chemical substance, Company: Ge, Coordination complex, energy, energy difference, Entertainment/Culture, Historical, Hydrogen bond, Isomer, Isomerism, Length, Molecular geometry, Organic chemistry, results of a search, search both bond stretch isomers, SN
Posted in crystal_structure_mining | 1 Comment »
Wednesday, January 20th, 2016
The original strategic objective of my PhD researches in 1972-74 was to explore how primary kinetic hydrogen isotope effects might be influenced by the underlying structures of the transition states involved. Earlier posts dealt with how one can construct quantum-chemical models of these transition states that fit the known properties of the reactions. Now, one can reverse the strategy by computing the expected variation with structure to see if anything interesting might emerge, and then if it does, open up the prospect of further exploration by experiment. Here I will use the base-catalysed enolisation of 1,3-dimethylindolin-2-ones and the decarboxylation of 3-indole carboxylates to explore this aspect.
(more…)
Tags:aqueous solution, Brian Challis, can construct quantum-chemical models, computed free energy barrier matches, Dan Singleton, free energy barrier, free energy barriers, Kinetic isotope effect, Organic chemistry, Physical organic chemistry, quantum-chemical models, supervisor
Posted in reaction mechanism | No Comments »
Thursday, December 24th, 2015
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then.  In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[1],[2] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[1],[2] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below. 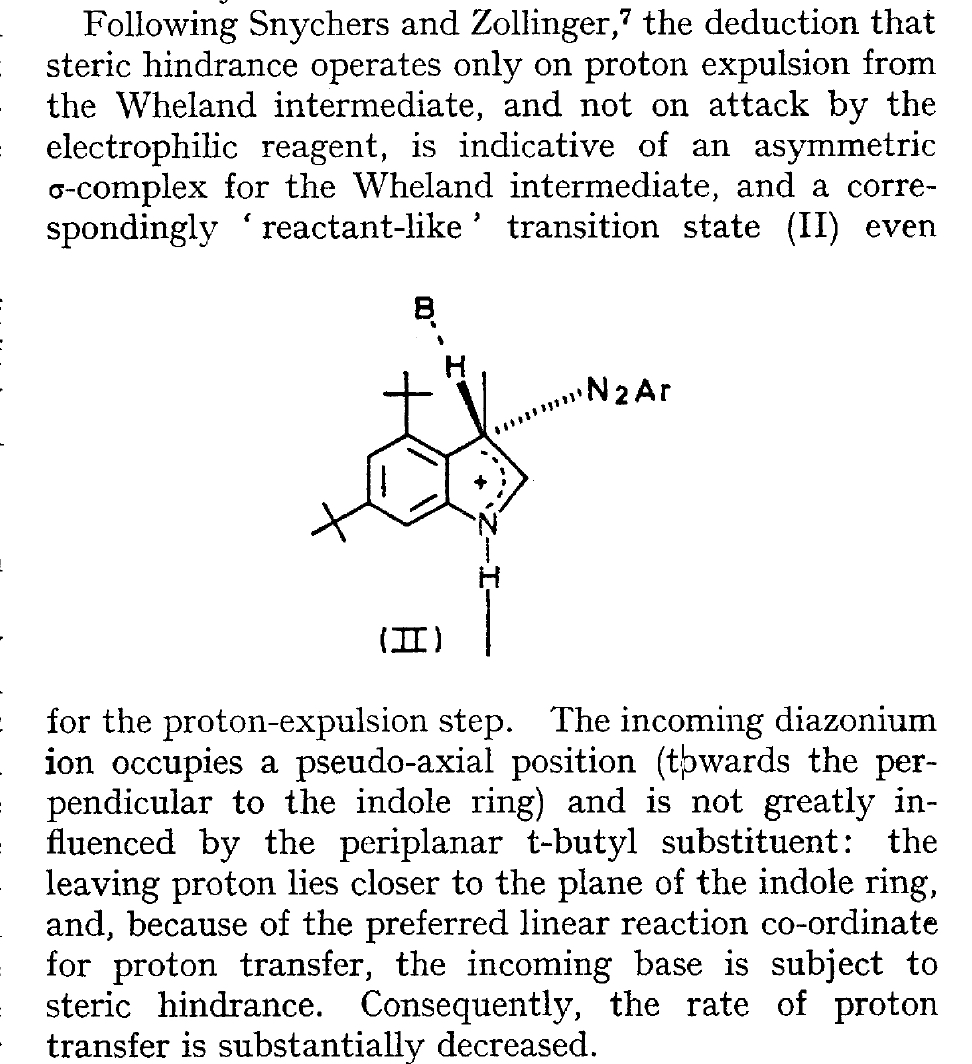 The main points of this argument were;
The main points of this argument were;
(more…)
References
-
B.C. Challis, and H.S. Rzepa, "The mechanism of diazo-coupling to indoles and the effect of steric hindrance on the rate-limiting step", Journal of the Chemical Society, Perkin Transactions 2, pp. 1209, 1975. http://dx.doi.org/10.1039/P29750001209
-
Rzepa, Henry S.., "Hydrogen transfer reactions of Indoles", 1974. http://dx.doi.org/10.5281/zenodo.18777
Tags:Butyl, chemical reactions, Historical, Indole, Interesting chemistry, Kinetic isotope effect, Organic chemistry, Physical organic chemistry, potential energy surfaces, relative energy
Posted in reaction mechanism | 1 Comment »
Friday, June 12th, 2015
In the preceding post, I discussed the reaction between mCPBA (meta-chloroperbenzoic acid) and cyclohexanone, resulting in Baeyer-Villiger oxidation via a tetrahedral intermediate (TI). Dan Singleton, in whose group the original KIE (kinetic isotope measurements) were made, has kindly pointed out on this blog that his was a mixed-phase reaction, and that mechanistic comparison with homogenous solutions may not be justified. An intriguing aspect of the (solution) mechanism would be whether the TI forms quickly and/or reversibly and what the position of any equilibrium between it and the starting ketone is. This reminded me of work we did some years ago,[1] and here I discuss that.
(more…)
References
-
A.M. Lobo, M.M. Marques, S. Prabhakar, and H.S. Rzepa, "Tetrahedral intermediates formed by nitrogen and oxygen attack of aromatic hydroxylamines on acetyl cyanide", The Journal of Organic Chemistry, vol. 52, pp. 2925-2927, 1987. http://dx.doi.org/10.1021/jo00389a050
Tags:Anomer, Anomeric effect, Carbohydrate chemistry, Carbohydrates, Chemistry, Dan Singleton, homogenous solutions, Ketone, Meta-Chloroperoxybenzoic acid, Organic chemistry, Tetrahedral carbonyl addition compound
Posted in reaction mechanism | 2 Comments »
In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[1],[2] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
 The main points of this argument were;
The main points of this argument were;