Posts Tagged ‘operating systems’
Saturday, July 19th, 2014
Whilst clusters of carbon atoms are well-known, my eye was caught by a recent article describing the detection of a cluster of boron atoms, B40 to be specific.[1] My interest was in how the σ and π-electrons were partitioned. In a C40, one can reliably predict that each carbon would contribute precisely one π-electron. But boron, being more electropositive, does not always play like that. Having one electron less per atom, one might imagine that a fullerene-like boron cluster would have no π-electrons. But the element has a propensity[2] to promote its σ-electrons into the π-manifold, leaving a σ-hole. So how many π-electrons does B40 have? These sorts of clusters are difficult to build using regular structure editors, and so coordinates are essential. The starting point for a set of coordinates with which to compute a wavefunction was the supporting information. Here is the relevant page: 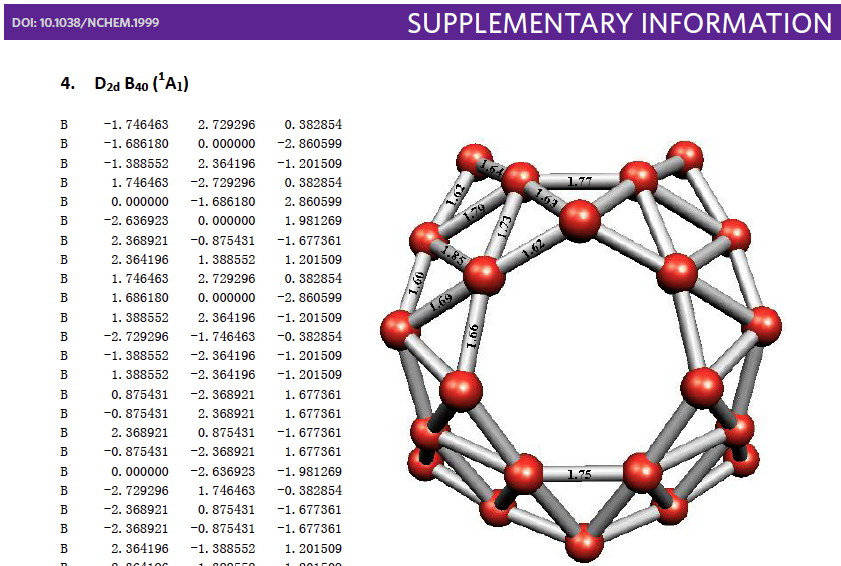 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
(more…)
References
- H. Zhai, Y. Zhao, W. Li, Q. Chen, H. Bai, H. Hu, Z.A. Piazza, W. Tian, H. Lu, Y. Wu, Y. Mu, G. Wei, Z. Liu, J. Li, S. Li, and L. Wang, "Observation of an all-boron fullerene", Nature Chemistry, vol. 6, pp. 727-731, 2014. https://doi.org/10.1038/nchem.1999
- H.S. Rzepa, "The distortivity of π-electrons in conjugated boron rings", Physical Chemistry Chemical Physics, vol. 11, pp. 10042, 2009. https://doi.org/10.1039/b911817a
Tags:Acrobat, Adobe, chemical markup, DOS, operating systems, PDF, pence, Unix
Posted in Chemical IT, Interesting chemistry | 2 Comments »
Monday, December 2nd, 2013
This is one of those topics that seems to crop up every three years or so. Since then, new versions of operating systems, new versions of programs, mobile devices and perhaps some progress?
(more…)
Tags:chemical data, chemical semantics, chemical structure diagrams, chemical structures, desktop operating systems, mature technology, mobile devices, much current software, operating system, operating systems, veritable word processor, Word, word processor, XML
Posted in Chemical IT | No Comments »
Tuesday, December 7th, 2010
Moving (chemical) data around in a manner which allows its (automated) use in whichever context it finds itself must be a holy grail for all scientists and chemists. I posted earlier on the fragile nature of molecular diagrams making the journey between the editing program used to create them (say ChemDraw) and the Word processor used to place them into a context (say Microsoft office), via an intermediate storage area known as the clipboard. The round trip between the Macintosh (OS X) versions of these programs had been broken a little while, but it is now fixed! A small victory. This blog reports what happened when such a Mac-created Word document is sent to someone using Microsoft Windows as an OS (or vice versa).
(more…)
Tags:Android, cellular telephone, chemical, chemical information, Chemical IT, content carrier, HTML, iPad, JPEG, Mac OS X, Macintosh, Microsoft, Microsoft Windows, opendata, operating systems, search engines, Web browser, word processor, XML
Posted in Chemical IT | No Comments »
 The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.
The coordinates are certainly there (that is not always the case), but you have to know a few tricks to make them usable.