Posts Tagged ‘energy’
Thursday, October 1st, 2015
A fascinating re-examination has appeared[1] of a reaction first published[2] in 1960 by Wittig and then[3] repudiated by him in 1964 since it could not be replicated by a later student. According to the new work, the secret to a successful replication seems to be the presence of traces of a nickel catalyst (originally coming from e.g. a nickel spatula?). In this recent article[1] a mechanism for the catalytic cycle is proposed. Here I thought I might explore this mechanism using calculations to see if any further insights might emerge.
(more…)
References
-
S.A. Künzi, J.M. Sarria Toro, T. den Hartog, and P. Chen, "Nickel‐Catalyzed Cyclopropanation with NMe4OTf and nBuLi", Angewandte Chemie International Edition, vol. 54, pp. 10670-10674, 2015. http://dx.doi.org/10.1002/anie.201505482
-
V. Franzen, and G. Wittig, "Trimethylammonium‐methylid als Methylen‐Donator", Angewandte Chemie, vol. 72, pp. 417-417, 1960. http://dx.doi.org/10.1002/ange.19600721210
-
G. Wittig, and D. Krauss, "Cyclopropanierungen bei Einwirkung von N‐Yliden auf Olefine", Justus Liebigs Annalen der Chemie, vol. 679, pp. 34-41, 1964. http://dx.doi.org/10.1002/jlac.19646790106
Tags:activation free energy barriers, Cambridge, energy, energy relative, Interesting chemistry, nickel-carbene product
Posted in reaction mechanism | No Comments »
Wednesday, June 10th, 2015
I have blogged before about the mechanism of this classical oxidation reaction. Here I further explore computed models, and whether they match the observed kinetic isotope effects (KIE) obtained using the natural-abundance method described in the previous post.
(more…)
Tags:ATM, Baeyer–Villiger oxidation, Chemistry, Dipole, energy, energy gradient, Kinetic isotope effect, Physical organic chemistry
Posted in reaction mechanism | 4 Comments »
Sunday, April 12th, 2015
Sodium borohydride is the tamer cousin of lithium aluminium hydride (LAH). It is used in aqueous solution to e.g. reduce aldehydes and ketones, but it leaves acids, amides and esters alone. Here I start an exploration of why it is such a different reducing agent.

(more…)
Tags:aqueous solution, Chemical bond, chemical bonding, Chemistry, Electronic effect, energy, final product, free energy barrier, Hydride, Hydrogen bond, immediate product, Lithium aluminium hydride, reduction
Posted in reaction mechanism | 2 Comments »
Friday, April 10th, 2015
Previously on this blog: modelling the reduction of cinnamaldehyde using one molecule of lithal shows easy reduction of the carbonyl but a high barrier at the next stage, the reduction of the double bond. Here is a quantum energetic exploration of what might happen when a second LAH is added to the brew (the usual ωB97XD/6-311+G(d,p)/SCRF=diethyl ether).
(more…)
Tags:computed free energy barrier, energy, energy surface, final product, flat energy potential, free energy, lower energy pathways, metal exchange, pence, potential energy surface, reduction, Yes
Posted in reaction mechanism | No Comments »
Saturday, February 14th, 2015
According to Guggemos, Slavicek and Kresin, about 5-6![1]. This is one of those simple ideas, which is probably quite tough to do experimentally. It involved blasting water vapour through a pinhole, adding HCl and measuring the dipole-moment induced deflection by an electric field. They found “evidence for a noticeable rise in the dipole moment occurring at n≈5–6“.
(more…)
References
-
N. Guggemos, P. Slavíček, and V.V. Kresin, "Electric Dipole Moments of Nanosolvated Acid Molecules in Water Clusters", Physical Review Letters, vol. 114, 2015. http://dx.doi.org/10.1103/PhysRevLett.114.043401
Tags:energy, gas phase models, Interesting chemistry, Java, pence, similar energy
Posted in reaction mechanism | No Comments »
Friday, January 23rd, 2015
Sometimes you come across a bond in chemistry that just shouts at you. This happened to me in 1989[1] with the molecule shown below. Here is its story and, 26 years later, how I responded.
(more…)
References
-
P. Camilleri, C.A. Marby, B. Odell, H.S. Rzepa, R.N. Sheppard, J.J.P. Stewart, and D.J. Williams, "X-Ray crystallographic and NMR evidence for a uniquely strong OH ? N hydrogen bond in the solid state and solution", Journal of the Chemical Society, Chemical Communications, pp. 1722, 1989. http://dx.doi.org/10.1039/C39890001722
Tags:energy, high chemical shifts, Historical, Interesting chemistry, New Hampshire, unusual chemical shift
Posted in Uncategorised | No Comments »
Saturday, November 1st, 2014
We are approaching 1 million recorded crystal structures (actually, around 716,000 in the CCDC and just over 300,00 in COD). One delight with having this wealth of information is the simple little explorations that can take just a minute or so to do. This one was sparked by my helping a colleague update a set of interactive lecture demos dealing with stereochemistry. Three of the examples included molecules where chirality originates in stereogenic centres with just three attached groups. An example might be a sulfoxide, for which the priority rule is to assign the lone pair present with atomic number zero. The issue then arises as to whether this centre is configurationally stable, i.e. does it invert in an umbrella motion slowly or quickly. My initial intention was to see if crystal structures could cast any light at all on this aspect.
(more…)
Tags:Chemical IT, energy, overlap/energy match, search definition
Posted in crystal_structure_mining | No Comments »
Thursday, June 26th, 2014
The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack.  My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder).
My first thought was to calculate the wavefunction and identify the location and energy (= electrophilicity) of the lone pairs (the presumed attractor of an electrophile). But a better more direct approach soon dawned. A search of the crystal structure database. Here is the search definition, with the C=O-E angle, the O-E distance and the N-C=O-E torsion defined (also specified for R factor < 5%, no errors and no disorder). 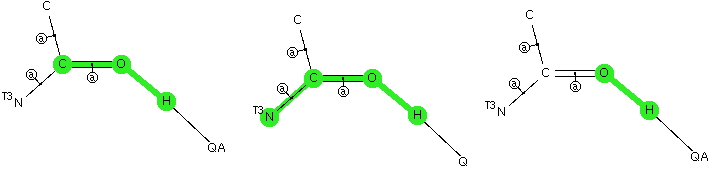 The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl)
The first plot is of the torsion vs the distance, for E = H-X (X=O,F, Cl) 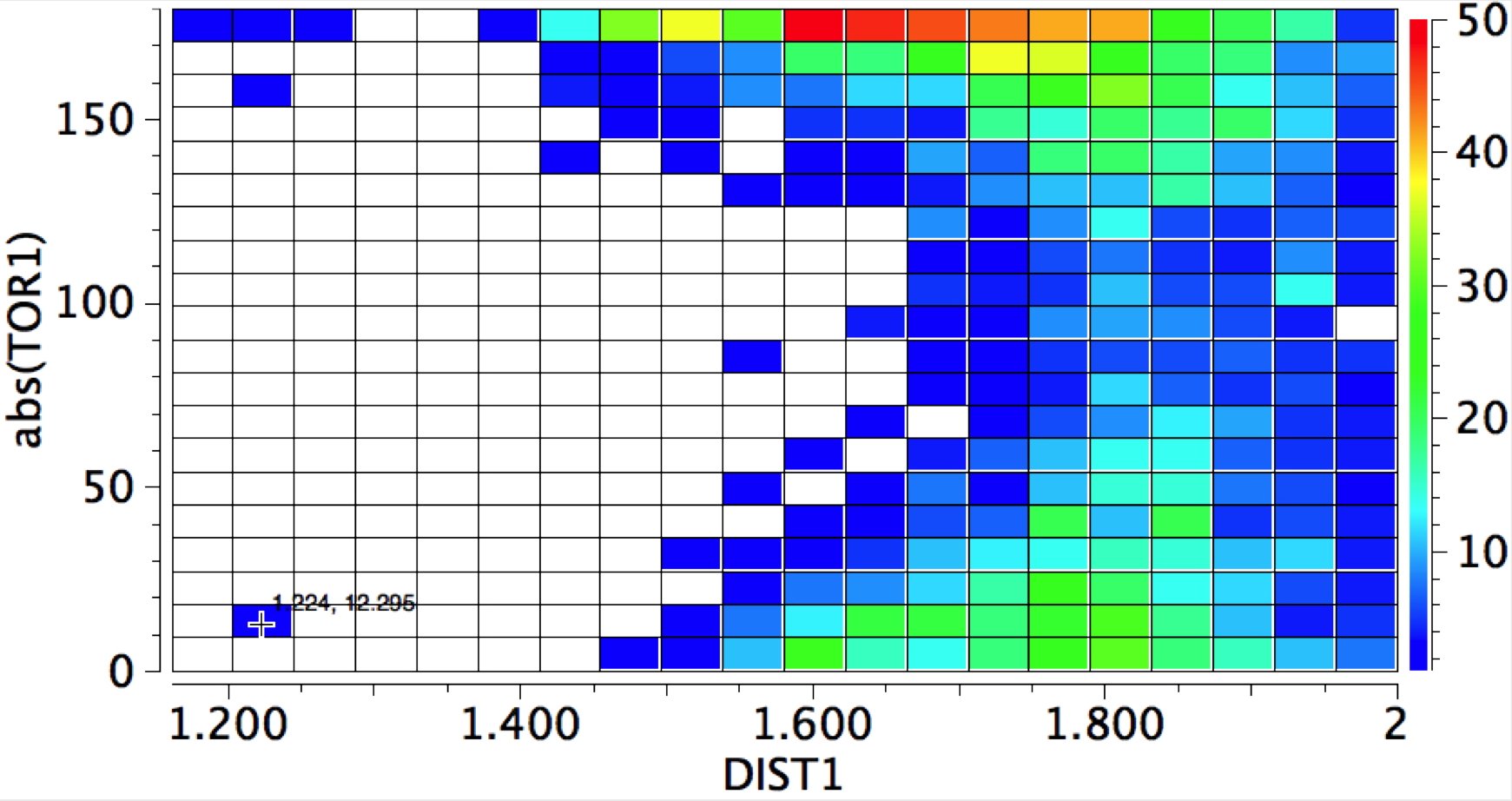
(more…)
Tags:electronics, energy, General, Interesting chemistry, metal results, metal-π-bond interactions, search definition
Posted in crystal_structure_mining | No Comments »
Sunday, April 20th, 2014
Ribulose-1,5-bisphosphate reacts with carbon dioxide to produce 3-keto-2-carboxyarabinitol 1,5-bisphosphate as the first step in the biochemical process of carbon fixation. It needs an enzyme to do this (Ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO) and lots of ATP (adenosine triphosphate, produced by photosynthesis). Here I ask what the nature of the uncatalysed transition state is, and hence the task that might be facing the catalyst in reducing the activation barrier to that of a facile thermal reaction. I present my process in the order it was done‡.
(more…)
Tags:1M solutions, carbon fixation, chair, chemist, energy, free energy, Interesting chemistry, low energy, low energy sink, lower energy conformation, lower energy isomer, Peter Medawar, phosphate
Posted in reaction mechanism | 1 Comment »
Tuesday, April 15th, 2014
The journal of chemical education can be a fertile source of ideas for undergraduate student experiments. Take this procedure for asymmetric epoxidation of an alkene.[1] When I first spotted it, I thought not only would it be interesting to do in the lab, but could be extended by incorporating some modern computational aspects as well.
(more…)
References
-
A. Burke, P. Dillon, K. Martin, and T.W. Hanks, "Catalytic Asymmetric Epoxidation Using a Fructose-Derived Catalyst", Journal of Chemical Education, vol. 77, pp. 271, 2000. http://dx.doi.org/10.1021/ed077p271
Tags:chemical education, energy, free energy, Interesting chemistry, Shi Fructose
Posted in reaction mechanism | 2 Comments »

