Posts Tagged ‘crystal structure search’
Thursday, January 3rd, 2019
There is emerging interest in cyclic conjugated molecules that happen to have triplet spin states and which might be expected to follow a 4n rule for aromaticity.[1] The simplest such system would be the triplet state of cyclobutadiene, for which a non or anti-aromatic singlet state is always found to be lower in energy. Here I explore some crystal structures containing this motif for possible insights.
(more…)
References
- A. Kostenko, B. Tumanskii, Y. Kobayashi, M. Nakamoto, A. Sekiguchi, and Y. Apeloig, "Spectroscopic Observation of the Triplet Diradical State of a Cyclobutadiene", Angewandte Chemie International Edition, vol. 56, pp. 10183-10187, 2017. https://doi.org/10.1002/anie.201705228
Tags:antiaromaticity, aromaticity, Baird's rule, Conjugated system, crystal structure search, energy, Hückel's rule, Nature, Physical organic chemistry, Physical sciences, search query, Triplet state
Posted in Interesting chemistry | No Comments »
Saturday, July 25th, 2015
I recently followed this bloggers trail; link1 → link2 to arrive at this delightful short commentary on atom-atom bonds in crystals[1] by Jack Dunitz. Here he discusses that age-old question (to chemists), what is a bond? Even almost 100 years after Gilbert Lewis’ famous analysis,[2] we continue to ponder this question. Indeed, quite a debate on this topic broke out in a recent post here. My eye was caught by one example in Jack's article: "The close stacking of planar anions, as occurs in salts of croconic acid …far from producing a lowering of the crystal energy, this stacking interaction in itself leads to an increase by several thousand kJ mol−1 arising from Coulombic repulsion between the doubly negatively charged anions" I thought I might explore this point a bit further in this post.
(more…)
References
- J.D. Dunitz, "Intermolecular atom–atom bonds in crystals?", IUCrJ, vol. 2, pp. 157-158, 2015. https://doi.org/10.1107/s2052252515002006
- G.N. Lewis, "THE ATOM AND THE MOLECULE.", Journal of the American Chemical Society, vol. 38, pp. 762-785, 1916. https://doi.org/10.1021/ja02261a002
Tags:Carbon, Cations, Chemical bond, control search, Croconic acid, crystal energy, crystal structure search, Gilbert Lewis, intermolecular search, Jack Dunitz, overall energy balance, Proton
Posted in Chemical IT, crystal_structure_mining, Interesting chemistry | 1 Comment »
Sunday, December 7th, 2014
Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[1]. 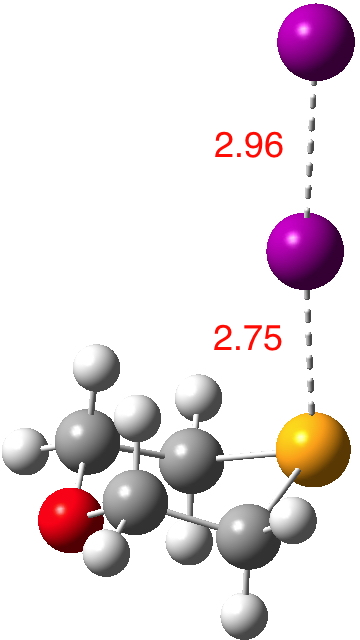 The features of interest include:
The features of interest include:
(more…)
References
- H. Maddox, and J.D. McCullough, "The Crystal and Molecular Structure of the Iodine Complex of 1-Oxa-4-selenacyclohexane, C<sub>4</sub>H<sub>8</sub>OSe.I<sub>2</sub>", Inorganic Chemistry, vol. 5, pp. 522-526, 1966. https://doi.org/10.1021/ic50038a006
Tags:chair, crystal structure search
Posted in crystal_structure_mining, Interesting chemistry | 7 Comments »
Saturday, November 29th, 2014
Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom (X, acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A. They are superficially surprising, since both D and X look like electron rich species. In fact the electron distribution around X-X (A=X) is highly anisotropic, with the electron rich distribution (the "donor") being in a torus encircling the bond, and an electron deficient region (the "acceptor") lying along the axis of the bond.
(more…)
Tags:crystal structure search, D. Note, frequent commentator, Paul Schleyer
Posted in crystal_structure_mining, Interesting chemistry, reaction mechanism | No Comments »

Halogen bonds: Part 1.
Saturday, November 29th, 2014Halogen bonds are less familiar cousins to hydrogen bonds. They are defined as non-covalent interactions (NCI) between a halogen atom (X, acting as a Lewis acid, in accepting electrons) and a Lewis base D donating electrons; D….X-A vs D…H-A. They are superficially surprising, since both D and X look like electron rich species. In fact the electron distribution around X-X (A=X) is highly anisotropic, with the electron rich distribution (the "donor") being in a torus encircling the bond, and an electron deficient region (the "acceptor") lying along the axis of the bond.
(more…)
Tags:crystal structure search, D. Note, frequent commentator, Paul Schleyer
Posted in crystal_structure_mining, Interesting chemistry, reaction mechanism | No Comments »