The 100th anniversary of G. N. Lewis’ famous electron pair theory of bonding is rapidly approaching in 2016 (DOI: 10.1021/ja02261a002). He set out a theory of bond types ranging from 1-6 electrons. The strongest bond recognized by this theory was the 6-electron triple bond, a good example of which occurs in dinitrogen, N2. In terms of valence electrons, nitrogen has an atomic configuration of 2s2, 2p3. Each atom has five electrons in total, some or all of which in principle could be used for forming bonds. An exploration of this motif across the entire periodic table is presented in part one of this blog.
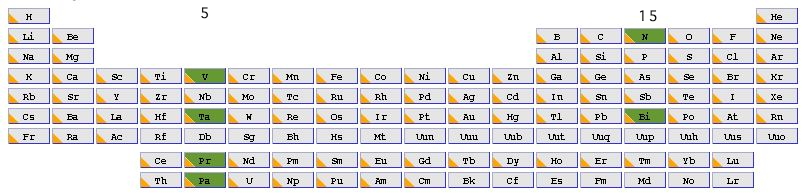
Nitrogen is in the main group 15, and the element at the bottom of this group is Bismuth (also with the same atomic configuration). We can then move to the corresponding column of the transition series, this time occupying group 5. The first examplar in this set, Vanadium has an atomic configuration of 3d3, 4s2, again five valence electrons, but now utilizing the d- rather than the p-shell of valence atomic orbitals (AOs). The final forage across the period table would land us with Pr and Pa, which occupy the lanthanide and actinide series respectively, and which have atomic configurations of 4f3, 6s2 and 5f2, 6d1 and 7s2 respectively. You can now see the theme developing; how does the bonding develop between two atoms that between them have ten valence electrons occupying molecular orbitals constructed from s, and then either p, d or f atomic orbitals. The next in that series, g atomic orbitals, are thought unlikely to have any chemical significance in the presently known periodic table.