Posts Tagged ‘chemical reactions’
Wednesday, July 25th, 2018
Consider the four reactions. The first two are taught in introductory organic chemistry as (a) a proton transfer, often abbreviated PT, from X to B (a base) and (b) a hydride transfer from X to A (an acid). The third example is taught as a hydrogen atom transfer or HAT from X to (in this example) O. Recently an article has appeared[1] citing an example of a fourth fundamental type (d), which is given the acronym cPCET which I will expand later. Here I explore this last type a bit further, in the context that X-H bond activations are currently a very active area of research.

(more…)
References
- J.E.M.N. Klein, and G. Knizia, "cPCET versus HAT: A Direct Theoretical Method for Distinguishing X–H Bond‐Activation Mechanisms", Angewandte Chemie International Edition, vol. 57, pp. 11913-11917, 2018. https://doi.org/10.1002/anie.201805511
Tags:chemical reactions, Chemistry, Deprotonation, Hydride, Hydrogen, Hydrogen atom abstraction, Proton, proton travel, Proton-coupled electron transfer, Technology/Internet
Posted in Interesting chemistry | No Comments »
Thursday, May 25th, 2017
It is a sign of the times that one travels to a conference well-connected. By which I mean email is on a constant drip-feed, with venue organisers ensuring each delegate receives their WiFi password even before their room key. So whilst I was at a conference espousing the benefits of open science, a nice example of open collaboration was initiated as a result of a received email.‡
(more…)
Tags:animation, chemical reactions, City: Cupertino, Company: Cupertino Elec, Company: Firefox Communic, Computer Hardware - NEC, computing, detective, Digital media, Drip, Electronic documents, Electronic publishing, Email, HTML, Imperial College, Linux, operating system, Password, Person Location, Steven Kirk, Technology/Internet, XML
Posted in Chemical IT | No Comments »
Thursday, December 24th, 2015
The BBC TV quiz series Mastermind was first broadcast in the UK in 1972, the same time I was starting to investigate the mechanism of diazocoupling to substituted indoles as part of my Ph.D. researches. The BBC program became known for the catch phrase I've started so I'll finish; here I will try to follow this precept with the project I started then.  In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[1],[2] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below.
In 1972, one measured the rates of chemical reactions to gain insights into the transition state kinetic model. To obtain more data, we used isotopes such as 2H or 3H, together with substituents such as R-t-butyl to modify the potential energy surfaces of the reactions by inducing steric effects.[1],[2] We found that the kinetics for this reaction were actually complex‡ (in part because of pH dependence) involving a Wheland intermediate (the formation of which is shown with red curly arrows above) followed by the collapse of this intermediate to the diazo-coupled product (blue arrows). Coupling to 2-methyl indole (R=X=H, R'=Me), 2-t-butyl indole (R=H, R'=t-butyl) and 4-methyl-2-t-butyl indole (R=Me, R'=t-butyl) revealed that the kinetic isotope effects induced by replacing H by D or T were "not apparent" (i.e. close to 1), the inference being that the rate constant k1 for those systems was slower than k2; the formation of the Wheland intermediate was rate determining (the rds) for the reaction. But with 2-methyl-4,6-di-t-butyl indole (R=t-butyl, R'=Me) this changed and a deuterium isotope effect of ~7 was observed. The rate determining proton removal from the Wheland intermediate k2 was now slower than k1. With 2,4,6-tri-t-butyl indole, we ended by noting that the reaction become almost too slow to observe and furthermore was accompanied by loss of a t-butyl cation as well as a proton. At this point we attempted to infer some transition state models consistent with these observations. Note that we had relatively little data with which to derive our 3D models (one needs to define a geometry using 3N-6 variables, along with its relative energy and force constants). The text and diagram of our attempt is shown below. 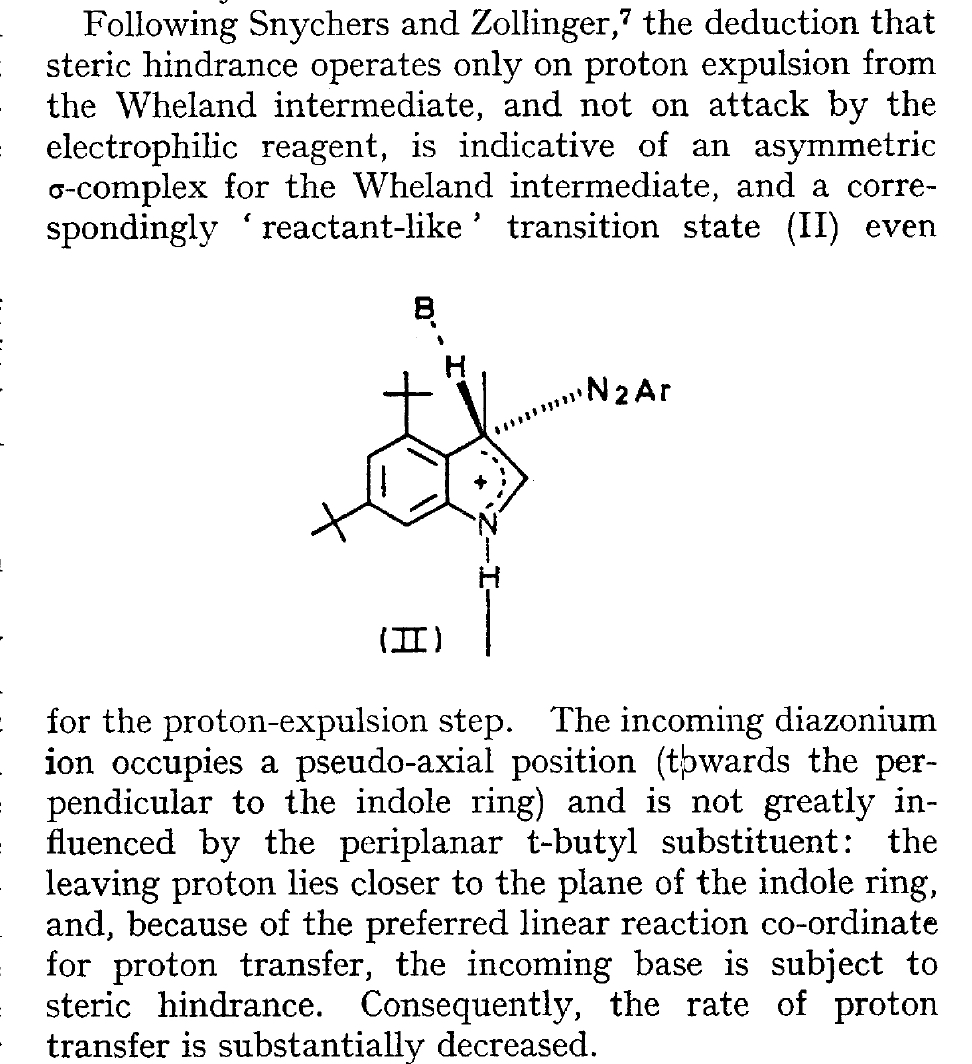 The main points of this argument were;
The main points of this argument were;
(more…)
References
- B.C. Challis, and H.S. Rzepa, "The mechanism of diazo-coupling to indoles and the effect of steric hindrance on the rate-limiting step", Journal of the Chemical Society, Perkin Transactions 2, pp. 1209, 1975. https://doi.org/10.1039/p29750001209
- H.S. Rzepa, "Hydrogen Transfer Reactions Of Indoles", Zenodo, 1974. https://doi.org/10.5281/zenodo.18777
Tags:Butyl, chemical reactions, Indole, Kinetic isotope effect, Organic chemistry, Physical organic chemistry, potential energy surfaces, relative energy
Posted in Historical, Interesting chemistry, reaction mechanism | 1 Comment »
 The main points of this argument were;
The main points of this argument were;