Posts Tagged ‘chemical’
Monday, April 1st, 2019
Members of the chemical FAIR data community have just met in Orlando (with help from the NSF, the American National Science Foundation) to discuss how such data is progressing in chemistry. There are a lot of themes converging at the moment. Thus this article[1] extolls the virtues of having raw NMR data available in natural product research, to which we added that such raw data should also be made FAIR (Findable, Accessible, Interoperable and Reusable) by virtue of adding rich metadata and then properly registering it so that it can be searched. These themes are combined in another article which made a recent appearance.[2]
(more…)
References
- J.B. McAlpine, S. Chen, A. Kutateladze, J.B. MacMillan, G. Appendino, A. Barison, M.A. Beniddir, M.W. Biavatti, S. Bluml, A. Boufridi, M.S. Butler, R.J. Capon, Y.H. Choi, D. Coppage, P. Crews, M.T. Crimmins, M. Csete, P. Dewapriya, J.M. Egan, M.J. Garson, G. Genta-Jouve, W.H. Gerwick, H. Gross, M.K. Harper, P. Hermanto, J.M. Hook, L. Hunter, D. Jeannerat, N. Ji, T.A. Johnson, D.G.I. Kingston, H. Koshino, H. Lee, G. Lewin, J. Li, R.G. Linington, M. Liu, K.L. McPhail, T.F. Molinski, B.S. Moore, J. Nam, R.P. Neupane, M. Niemitz, J. Nuzillard, N.H. Oberlies, F.M.M. Ocampos, G. Pan, R.J. Quinn, D.S. Reddy, J. Renault, J. Rivera-Chávez, W. Robien, C.M. Saunders, T.J. Schmidt, C. Seger, B. Shen, C. Steinbeck, H. Stuppner, S. Sturm, O. Taglialatela-Scafati, D.J. Tantillo, R. Verpoorte, B. Wang, C.M. Williams, P.G. Williams, J. Wist, J. Yue, C. Zhang, Z. Xu, C. Simmler, D.C. Lankin, J. Bisson, and G.F. Pauli, "The value of universally available raw NMR data for transparency, reproducibility, and integrity in natural product research", Natural Product Reports, vol. 36, pp. 35-107, 2019. https://doi.org/10.1039/c7np00064b
- A. Barba, S. Dominguez, C. Cobas, D.P. Martinsen, C. Romain, H.S. Rzepa, and F. Seoane, "Workflows Allowing Creation of Journal Article Supporting Information and Findable, Accessible, Interoperable, and Reusable (FAIR)-Enabled Publication of Spectroscopic Data", ACS Omega, vol. 4, pp. 3280-3286, 2019. https://doi.org/10.1021/acsomega.8b03005
Tags:American National Science Foundation, Bond length, ChemDraw, chemical, Chemistry, City: Orlando, Company: NSF, Force field, Intermolecular forces, Molecular geometry, National Science Foundation, natural product, Natural sciences, Orlando, Physical organic chemistry, Physical sciences, Quantum chemistry, Science and technology in the United States, Stereochemistry, steric energy, steric energy test, Strain, suitable free tool, unstable natural product, X-ray
Posted in Chemical IT | No Comments »
Monday, August 1st, 2016
In March, I posted from the ACS meeting in San Diego on the topic of Research data: Managing spectroscopy-NMR, and noted a talk by MestreLab Research on how a tool called Mpublish in the forthcoming release of their NMR analysis software Mestrenova could help. With that release now out, the opportunity arose to test the system.
(more…)
Tags:Acrobat, analysis software, chemical, Chemistry, City: San Diego, format type chemical/x-mnpub, media type, Mestrenova, non-commercial open software packages, Nuclear magnetic resonance, Nuclear magnetic resonance spectra database, Nuclear magnetic resonance spectroscopy, PDF, public key, Science, Scientific method, spectroscopy, Technology/Internet
Posted in Chemical IT | 3 Comments »
Monday, March 7th, 2016
Tags:Academic publishing, chemical, chemical information division, Chemical nomenclature, chemical structures, Chemical substance, chemical/x-wavefunction, Cheminformatics, City: San Diego, content media, data repository search, format type chemical/x-* , Identifiers, Imperial College, Imperial College London, International Chemical Identifier, JSON, media types, multipurpose internet media extensions, ORCiD, PDF, potential such systems, research data management, Search queries, Technical communication, Technology/Internet
Posted in Chemical IT | 2 Comments »
Saturday, July 5th, 2014
I was lucky enough to attend the announcement made in 2012 of the discovery of the Higgs Boson. It consisted of a hour-long talk mostly about statistics, and how the particle physics community can only claim a discovery when their data has achieved a 5σ confidence level. This represents a 1 in 3.5 million probability of the result occurring by chance. I started thinking: how much chemistry is asserted at that level of confidence? Today, I read Steve Bachrach’s post on the structure of Citrinalin B and how “use of Goodman’s DP4 method indicates a 100% probability that the structure of citrinalin B is (the structure below)”. Wow, that is even higher than the physicists. Of course, 100% has been obtained by rounding 99.7 (3σ is 99.73%) or whatever (this is one number that should never be so rounded!). 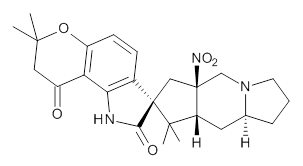 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
(more…)
References
- E.V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E.F. Pimenta, D.K. Romney, M.W. Lodewyk, D.E. Williams, R.J. Andersen, S.J. Miller, D.J. Tantillo, R.G.S. Berlinck, and R. Sarpong, "Total synthesis and isolation of citrinalin and cyclopiamine congeners", Nature, vol. 509, pp. 318-324, 2014. https://doi.org/10.1038/nature13273
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao, and J. Kobayashi, "Absolute Stereochemistry of Citrinadins A and B from Marine-Derived Fungus", The Journal of Organic Chemistry, vol. 70, pp. 9430-9435, 2005. https://doi.org/10.1021/jo051499o
- F. Cherblanc, Y. Lo, E. De Gussem, L. Alcazar‐Fuoli, E. Bignell, Y. He, N. Chapman‐Rothe, P. Bultinck, W.A. Herrebout, R. Brown, H.S. Rzepa, and M.J. Fuchter, "On the Determination of the Stereochemistry of Semisynthetic Natural Product Analogues using Chiroptical Spectroscopy: Desulfurization of Epidithiodioxopiperazine Fungal Metabolites", Chemistry – A European Journal, vol. 17, pp. 11868-11875, 2011. https://doi.org/10.1002/chem.201101129
- F.L. Cherblanc, Y. Lo, W.A. Herrebout, P. Bultinck, H.S. Rzepa, and M.J. Fuchter, "Mechanistic and Chiroptical Studies on the Desulfurization of Epidithiodioxopiperazines Reveal Universal Retention of Configuration at the Bridgehead Carbon Atoms", The Journal of Organic Chemistry, vol. 78, pp. 11646-11655, 2013. https://doi.org/10.1021/jo401316a
Tags:chemical, Reading, Steve Bachrach, X-ray
Posted in General | 2 Comments »
Friday, October 11th, 2013
In 1993-1994, when the Web (synonymous in most minds now with the Internet) was still young, the pace of progress was so rapid that some wag worked out that one “web-year” was like a dog-year, worth about 7 years of normal human time. So in this respect, 1994 is now some 133 web-years ago. Long enough for an archaeological excavation.
(more…)
Tags:chemical, Peter Murray-Rust, Web-pages, web-years
Posted in Chemical IT | 1 Comment »
Saturday, January 19th, 2013
The transient π-complex formed during the “[5,5]” sigmatropic rearrangement of protonated N,O-diphenyl hydroxylamine can be (formally) represented as below, namely the interaction of a six-π-electron aromatic ring (the phenoxide anion 2) with a four-π-electron phenyl dication-anion pair 1. Can one analyse this interaction in terms of aromaticity?
(more…)
Tags:chemical, Michael Dewar
Posted in Interesting chemistry | 1 Comment »
Tuesday, July 12th, 2011
In 1986 or so, molecular modelling came of age. Richard Counts, who ran an organisation called QCPE (here I had already submitted several of the program codes I had worked on) had a few years before contacted me to ask for my help with his Roadshow. He had started these in the USA as a means of promoting QCPE, which was the then main repository of chemistry codes, and as a means of showing people how to use the codes. My task was to organise a speakers list, the venue being in Oxford in a delightful house owned by the university computing services. Access to VAX computers was provided, via VT100 terminals. Amazingly, these terminals could do very primitive molecular graphics (using delightfully named escape codes, which I learnt to manipulate).
(more…)
Tags:3D graphics, antimalarial, antimalarial pharmaceutical molecule, chemical, co-processor, Florida, Gainesville, George Purvis, Halofantrine, haptic device, Historical, HTML, Macintosh, Mike Webb, Ohio, Oxford, pharmaceutical, Richard Counts, security guard, tangled web, Tektronix, United Kingdom, United States, university computing services, University of Florida
Posted in Chemical IT, Interesting chemistry | 4 Comments »
Friday, July 8th, 2011
As a personal retrospective of my use of computers (in chemistry), the Macintosh plays a subtle role. (more…)
Tags:Apple computer, Appletalk, Australia, chemical, copy-editor, ethernet, Eudora, Fibre optic, GBP, hand-drawn chemical diagrams, Historical, IBM, laser printer, Mac OS X, Macintosh, megabit network, mouse pointing device, multigate routers, operating system, Pirelli, Pittsburgh, proper network, Royal Society of Chemistry, stereographics device, telephone negotiation, Tim Berners-Lee, Webster multigate routers, XML
Posted in Chemical IT | 3 Comments »
Wednesday, June 15th, 2011
It is not often that an article on the topic of illusion and deception makes it into a chemical journal. Such is addressed (DOI: 10.1002/anie.201102210) in no less an eminent journal than Angew Chemie. The illusion (or deception if you will) actually goes to the heart of how we represent three-dimensional molecules in two dimensions, and the meanings that may be subverted by doing so. A it happens, it is also a recurring theme of this particular blog, which is the need to present chemistry with data for all three dimensions fully intact (hence the Click for 3D captions which often appear profusely here).
(more…)
Tags:chemical, chemical journal, chemist, M. C. Escher, Tutorial material
Posted in Chemical IT, Interesting chemistry | 2 Comments »
Wednesday, December 22nd, 2010
If you visit this blog you will see a scientific discourse in action. One of the commentators there notes how they would like to access some data made available in a journal article via the (still quite rare) format of an interactive table, but they are not familiar with how to handle that kind of data (file). The topic in question deals with various kinds of (chemical) data, including crystallographic information, computational modelling, and spectroscopic parameters. It could potentially deal with much more. It is indeed difficult for any one chemist to be familiar with how data is handled in such diverse areas. So I thought I would put up a short tutorial/illustration in this post of how one might go about extracting and re-using data from this one particular source.
(more…)
Tags:chemical, chemical journals, chemist, opendata, RDF, semantic web, software tools, suitable processing programs, XML
Posted in Chemical IT, Interesting chemistry | 7 Comments »
 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes