Posts Tagged ‘Carbohydrates’
Monday, August 8th, 2016
The previous post contained an exploration of the anomeric effect as it occurs at an atom centre X for which the effect is manifest in crystal structures. Here I quantify the effect, by selecting the test molecule MeO-X-OMe, where X is of two types:
(more…)
Tags:Anomer, Anomeric effect, Atomic orbital, Carbohydrate chemistry, Carbohydrates, Chemical bond, chemical bonding, Chemistry, Hydrogen bond, interaction energy, Lone pair, Physical organic chemistry, Quantum chemistry
Posted in Interesting chemistry | No Comments »
Saturday, August 6th, 2016
In the last few posts, I have explored the anomeric effect as it occurs at an atom centre X. Here I try to summarise the atoms for which the effect is manifest in crystal structures.
(more…)
Tags:Acetals, Alkane stereochemistry, Anomer, Anomeric effect, Atomic orbital, Carbohydrate chemistry, Carbohydrates, Chemical bond, Chemistry, interaction energy, Lone pair, Physical organic chemistry, Stereochemistry
Posted in crystal_structure_mining, Interesting chemistry | No Comments »
Friday, July 8th, 2016
The previous post looked at anomeric effects set up on centres such as B, Si or P, and involving two oxygen groups attached to these atoms. Here I vary the attached groups to include either one or two nitrogen atoms.[1]
(more…)
References
- H. Rzepa, "Anomeric effects at carbon, involving lone pairs originating from one or two nitrogens", 2016. https://doi.org/10.14469/hpc/936
Tags:Anomer, Anomeric effect, Carbohydrate chemistry, Carbohydrate conformation, Carbohydrates, Chemistry, Nitrogen
Posted in crystal_structure_mining | No Comments »
Friday, July 1st, 2016
The anomeric effect occurs at 4-coordinate (sp3) carbon centres carrying two oxygen substituents and involves an alignment of a lone electron pair on one oxygen with the adjacent C-O σ*-bond of the other oxygen. Here I explore whether other centres can exhibit the phenomenon. I start with 4-coordinate boron, using the crystal structure search definition below (along with R < 0.1, no disorder, no errors).[1]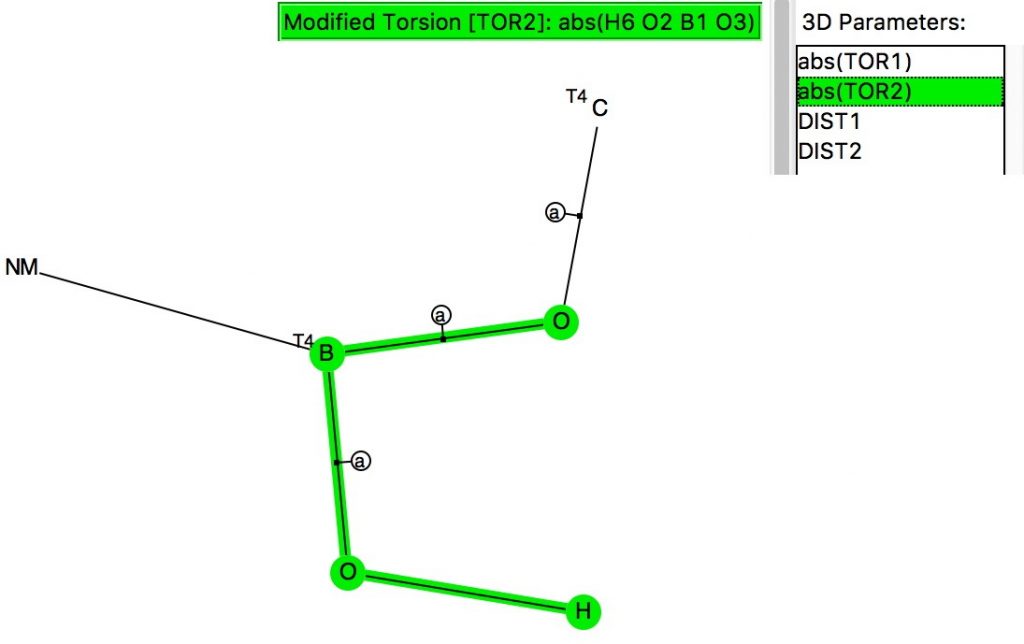
(more…)
References
- H. Rzepa, "Anomeric effects at boron, silicon and phosphorus.", 2016. https://doi.org/10.14469/hpc/696
Tags:Acetals, Alkane stereochemistry, Anomer, Anomeric effect, Bond length, Boron, Carbohydrate, Carbohydrate chemistry, Carbohydrates, crystal structure search definition, Ester, Physical organic chemistry, Stereochemistry
Posted in crystal_structure_mining | No Comments »
Monday, May 30th, 2016
This is a follow-up to one aspect of the previous two posts dealing with nucleophilic substitution reactions at silicon. Here I look at the geometries of 5-coordinate compounds containing as a central atom 4A = Si, Ge, Sn, Pb and of the specific formula C34AO2 with a trigonal bipyramidal geometry. This search arose because of a casual comment I made in the earlier post regarding possible cooperative effects between the two axial ligands (the ones with an angle of ~180 degrees subtended at silicon). Perhaps the geometries might expand upon this comment?
(more…)
Tags:Anomer, Anomeric effect, Carbohydrate chemistry, Carbohydrates, Ligand, Molecular geometry, Physical organic chemistry, Stereochemistry, Stereoelectronic effect, Trigonal bipyramidal molecular geometry
Posted in Chemical IT, crystal_structure_mining | 3 Comments »
Thursday, August 27th, 2015
The anomeric effect is best known in sugars, occuring in sub-structures such as RO-C-OR. Its origins relate to how the lone pairs on each oxygen atom align with the adjacent C-O bonds. When the alignment is 180°, one oxygen lone pair can donate into the C-O σ* empty orbital and a stabilisation occurs. Here I explore whether crystal structures reflect this effect.
(more…)
Tags:Alkane stereochemistry, Anomeric effect, Carbohydrate chemistry, Carbohydrates, Carbon–oxygen bond, Chemical bond, Ether, Lone pair, Physical organic chemistry, Quantum chemistry, Stereochemistry, Technology/Internet
Posted in Chemical IT, crystal_structure_mining | No Comments »
Friday, June 12th, 2015
In the preceding post, I discussed the reaction between mCPBA (meta-chloroperbenzoic acid) and cyclohexanone, resulting in Baeyer-Villiger oxidation via a tetrahedral intermediate (TI). Dan Singleton, in whose group the original KIE (kinetic isotope measurements) were made, has kindly pointed out on this blog that his was a mixed-phase reaction, and that mechanistic comparison with homogenous solutions may not be justified. An intriguing aspect of the (solution) mechanism would be whether the TI forms quickly and/or reversibly and what the position of any equilibrium between it and the starting ketone is. This reminded me of work we did some years ago,[1] and here I discuss that.
(more…)
References
- A.M. Lobo, M.M. Marques, S. Prabhakar, and H.S. Rzepa, "Tetrahedral intermediates formed by nitrogen and oxygen attack of aromatic hydroxylamines on acetyl cyanide", The Journal of Organic Chemistry, vol. 52, pp. 2925-2927, 1987. https://doi.org/10.1021/jo00389a050
Tags:Anomer, Anomeric effect, Carbohydrate chemistry, Carbohydrates, Chemistry, Dan Singleton, homogenous solutions, Ketone, Meta-Chloroperoxybenzoic acid, Organic chemistry, Tetrahedral carbonyl addition compound
Posted in reaction mechanism | 2 Comments »
