The previous post contained an exploration of the anomeric effect as it occurs at an atom centre X for which the effect is manifest in crystal structures. Here I quantify the effect, by selecting the test molecule MeO-X-OMe, where X is of two types:
Posts Tagged ‘Anomeric effect’
A periodic table for anomeric centres, this time with quantified interactions.
Monday, August 8th, 2016A periodic table for anomeric centres.
Saturday, August 6th, 2016In the last few posts, I have explored the anomeric effect as it occurs at an atom centre X. Here I try to summarise the atoms for which the effect is manifest in crystal structures.
Anomeric effects at carbon involving lone pairs originating from one or two nitrogens.
Friday, July 8th, 2016The previous post looked at anomeric effects set up on centres such as B, Si or P, and involving two oxygen groups attached to these atoms. Here I vary the attached groups to include either one or two nitrogen atoms.[1]
References
- H. Rzepa, "Anomeric effects at carbon, involving lone pairs originating from one or two nitrogens", 2016. https://doi.org/10.14469/hpc/936
Anomeric effects at boron, silicon and phosphorus.
Friday, July 1st, 2016
The anomeric effect occurs at 4-coordinate (sp3) carbon centres carrying two oxygen substituents and involves an alignment of a lone electron pair on one oxygen with the adjacent C-O σ*-bond of the other oxygen. Here I explore whether other centres can exhibit the phenomenon. I start with 4-coordinate boron, using the crystal structure search definition below (along with R < 0.1, no disorder, no errors).[1]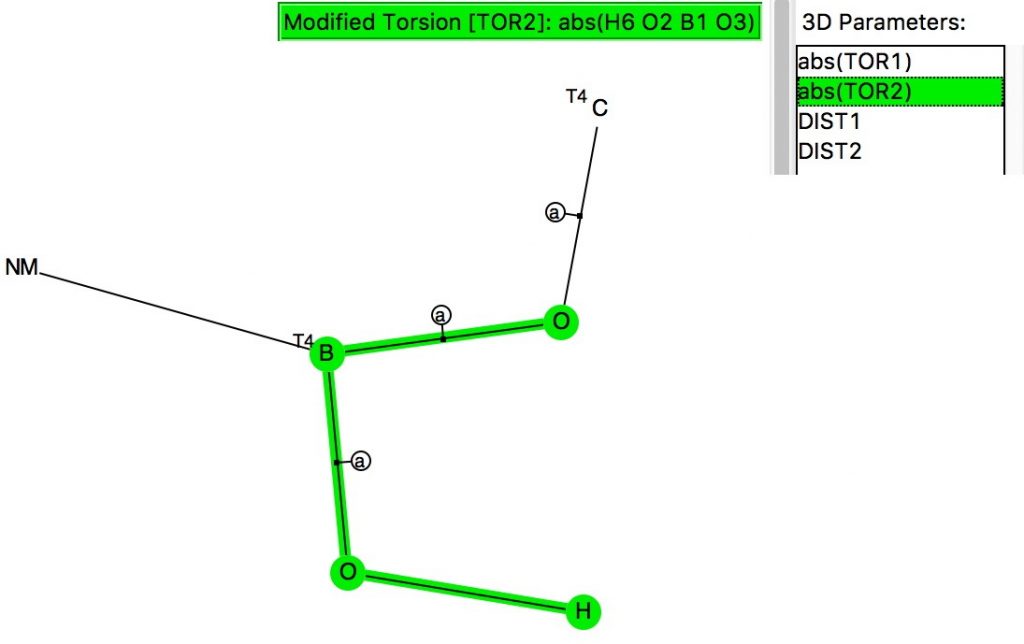
References
- H. Rzepa, "Anomeric effects at boron, silicon and phosphorus.", 2016. https://doi.org/10.14469/hpc/696
The geometries of 5-coordinate compounds of group 14 elements.
Monday, May 30th, 2016This is a follow-up to one aspect of the previous two posts dealing with nucleophilic substitution reactions at silicon. Here I look at the geometries of 5-coordinate compounds containing as a central atom 4A = Si, Ge, Sn, Pb and of the specific formula C34AO2 with a trigonal bipyramidal geometry. This search arose because of a casual comment I made in the earlier post regarding possible cooperative effects between the two axial ligands (the ones with an angle of ~180 degrees subtended at silicon). Perhaps the geometries might expand upon this comment?
Tunable bonds looked at in a different way
Sunday, July 11th, 2010The title of this post merges those of the two previous ones. The tunable C-Cl bond brought about in the molecule tris(amino)chloromethane by anomeric effects will be probed using the Laplacian of the electronic density.