This story starts with a calixarene, a molecule (suitably adorned with substituents) frequently used as a host to entrap a guest and perchance make the guest do something interesting. Such a calixarene was at the heart of a recent story where an attempt was made to induce it to capture cyclobutadiene in its cavity.
Posts Tagged ‘animation’
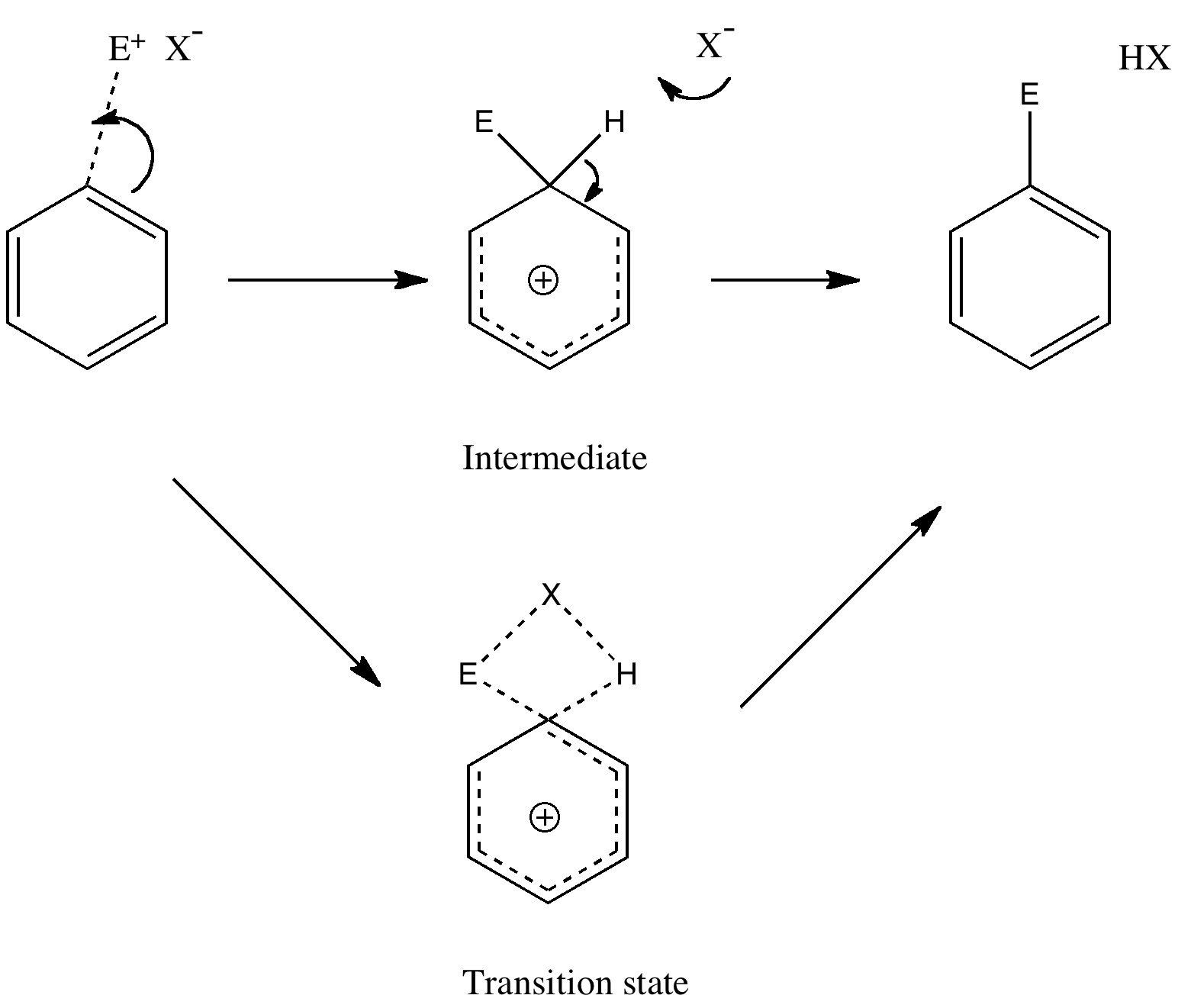
The oldest reaction mechanism: updated!
Tuesday, September 14th, 2010Unravelling reaction mechanisms is thought to be a 20th century phenomenon, coincident more or less with the development of electronic theories of chemistry. Hence electronic arrow pushing as a term. But here I argue that the true origin of this immensely powerful technique in chemistry goes back to the 19th century. In 1890, Henry Armstrong proposed what amounts to close to the modern mechanism for the process we now know as aromatic electrophilic substitution [1]. Beyond doubt, he invented what is now known as the Wheland Intermediate (about 50 years before Wheland wrote about it, and hence I argue here it should really be called the Armstrong/Wheland intermediate). This is illustrated (in modern style) along the top row of the diagram.
References
- "Proceedings of the Chemical Society, Vol. 6, No. 85", Proceedings of the Chemical Society (London), vol. 6, pp. 95, 1890. http://dx.doi.org/10.1039/PL8900600095
Bio-renewable green polymers: Stereoinduction in poly(lactic acid)
Saturday, July 24th, 2010Lactide is a small molecule made from lactic acid, which is itself available in large quantities by harvesting plants rather than drilling for oil. Lactide can be turned into polymers with remarkable properties, which in turn degrade down easily back to lactic acid. A perfect bio-renewable material!
Tunable bonds
Saturday, July 3rd, 2010Car transmissions come in two types, ones with fixed ratio gears, and ones which are continuously variable. When it comes to chemical bonds, we tend to think of them as being very much of the first type. Bonds come in fixed ratios; single, aromatic, double, triple, etc. OK, they do vary, but the variations are assumed as small perturbations on the basic form. Take for example the molecule shown below. The bonds as shown are all clearly single (the wedge and hashed bond are merely stereochemical notations). No-one would really think of drawing this molecule in any other way, and this idea of the transferability of bonds between molecules (all double bonds react in specific ways which are different from single bonds, and they also have characteristic spectroscopic properties, etc) is what allows molecules to be classified.
Chemistry with a super-twist: A molecular trefoil knot, part 2.
Tuesday, June 1st, 2010A conjugated, (apparently) aromatic molecular trefoil might be expected to have some unusual, if not extreme properties. Here some of these are explored. (more…)
The structure of the hydrogen ion in water.
Sunday, February 21st, 2010Stoyanov, Stoyanova and Reed recently published on the structure of the hydrogen ion in water. Their model was H(H2O)n+, where n=6 (DOI: 10.1021/ja9101826). This suggestion was picked up by Steve Bachrach on his blog, where he added a further three structures to the proposed list, and noted of course that with this type of system there must be a fair chance that the true structure consists of a well-distributed Boltzmann population of a number of almost iso-energetic forms.
The conformation of cyclohexane
Thursday, January 28th, 2010Like benzene, its fully saturated version cyclohexane represents an icon of organic chemistry. By 1890, the structure of planar benzene was pretty much understood, but organic chemistry was still struggling somewhat to fully embrace three rather than two dimensions. A grand-old-man of organic chemistry at the time, Adolf von Baeyer, believed that cyclohexane too was flat, and what he said went. So when a young upstart named Hermann Sachse suggested it was not flat, and furthermore could exist in two forms, which we now call chair and boat, no-one believed him. His was a trigonometric proof, deriving from the tetrahedral angle of 109.47 at carbon, and producing what he termed strainless rings.
Chemical intimacy: Ion pairs in carbocations
Monday, January 11th, 2010The scheme below illustrates one of the iconic reactions in organic chemistry. It is a modern representation of Meerwein’s famous experiment from which he inferred a carbocation intermediate, deduced from studying the rate of enantiomerization of isobornyl chloride when treated with the Lewis acid SnCl4.